Potential effects of miR‑146 expression in relation to malondialdehyde as a biomarker for oxidative damage in patients with breast cancer
- Authors:
- Published online on: January 13, 2023 https://doi.org/10.3892/wasj.2023.187
- Article Number: 10
-
Copyright: © Al-Khafaji et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
According to Global Cancer Statistics 2020, breast cancer is the most prevalent malignancy and is one of the top known causes of cancer-related mortality among females worldwide (1). Even in the general Iraqi population, breast cancer has been the highest-ranked malignancy since 1986(2), and the latest Cancer Registry in Iraq has observed 7,515 new breast cancer cases in 2020, accounting for 37.9% of the total reported cancer cases. Furthermore, breast cancer is the most common malignant tumor among Iraqi women (3).
Moreover, well-known risk factors (such as ionizing radiation, having a family history of cancer, alcoholism, obesity, hormone therapy during menopause, an older age, etc.), and several other factors, such as genetic predisposition from parents (4) along with epigenetic factors, have been recently reported to be associated with breast cancer development (5).
Breast carcinogens originate from a small cellular population known as breast cancer stem cells (BCSCs), that have a distinct molecular signature (6), and whose origin continues to be controversial. Some studies have reported that BCSCs originate from mammary stem cells or progenitor cells (7-9), while others have demonstrated that they arise from differentiated mammary cells (10-12). A low proportion (5-10%) of breast cancer cases may be associated with inherited mutations in the BRCA genes, which occur only in women (13,14).
MicroRNAs (miRNAs/miRs) are small, non-coding, single stranded (18-22 nucleotides in length) RNA molecules that function to regulate the post-transcriptional machinery of gene expression (15). Previous research has reported that miRNAs are involved in tumorigenesis (16), playing a critical role in the genesis and progression of breast cancer (17), potentially through the regulation of BCSCs (18). miRNAs play a crucial role in regulating the biological functions of a cell on a variety of levels. A number of diseases, including cancer, have been associated with miRNAs. There has been a rapid increase in interest in miR-146a in particular, as a modulator of differentiation and function as well as innate and adaptive immunity. miR-146a has been implicated in regulating a number of key cellular functions; thus, there are various types of tumors (papillary thyroid carcinoma, breast cancer and cervical cancer) with a dysregulated expression of miR-146a (19). Evidently, miR-146 levels have been shown to be consistently higher in breast cancer cells that exhibit tumor aggressive characteristics, among a variety of molecular subcategories of breast malignancy, along with an observed peak enrichment in BCSCs (20).
As regards the molecular mechanisms of miR-146 in breast cancer, it was previously demonstrated that miR-146a-5p overexpression in MCF-7 cells led to an increased proliferation, and the low expression miR-146a-5p in MCF-7 cells led to a decreased proliferation (21). By analyzing bioinformatics data and detecting fluorescent reporter genes, miR-146a-5p was identified as a gene target for BRCA1. Breast cancer tissue and MCF-7 cells expressing miR-146a-5p may regulate the proliferation of the cancer cell line via BRCA1(21). Another study also found that the miR-146a expression levels were significantly higher in breast cancers with various pathological classifications, while non-metastatic protein 23 H1 (NM23-H1) expression levels were significantly lower, which were closely correlated (22). In a breast cancer cell line, miR-146 and NM23-H1 were verified to have target regulatory associations by double luciferase reporter gene assays. miR-146a was closely related to the proliferation and metastasis of breast cancer. Additionally, miR-146a targeted NM23-H1 in vivo to promote breast cancer growth (22).
Malondialdehyde (MDA) is an organic compound that occurs naturally and its determination in blood plasma or tissues functions as a predictive marker of oxidative stress (23,24). When biomolecule peroxidation occurs, a number of carcinogenic and mutagenic factors are produced (25). Exploring the potential role of ectopic gene expression in patients with cancer has recently attracted the research interests of the authors (26-29). Despite the fact that a higher expression of miR-146 is associated with increased levels of MDA in normal tissues (30-32), to date, at least to the best of our knowledge, there are no reports available suggesting a similar association in malignant tissues. Therefore, the present study aimed to investigate the association between miR-146 expression and oxidative stress, as indicated by MDA levels, in breast tumorigenesis.
Subjects and methods
Subjects and sampling
Blood samples were collected from patients with breast cancer (n=30) and healthy women (age-matched controls; n=20), between January 3 to March 23, 2022. The inclusion criteria used to recruit individuals in the present study involved female patients diagnosed with stage I-III breast primary tumors aged 30-70 years. Control individuals included apparently healthy females with the same age range aforementioned. Males, patients with breast secondary tumors and those out of the age range mentioned above were, otherwise, excluded. Relevant ethics approval was obtained from the Biomedical Research Ethics Committee of the leading National Cancer Research Center at the University of Baghdad (reference no. NCRCEC/01/001). All individuals participating in the study were recruited from the Baghdad Teaching Hospital and Oncology Hospital, Baghdad, Iraq, after providing written informed consent. The recruitment process was carried out based on clinical/laboratory examinations and diagnoses by specialist doctors in the hospitals. A questionnaire was prepared to obtain patient information, including name, age and a family health history. The clinical information of all the study subjects is presented in Table I.
Blood samples were collected in VACUETTE® tubes, K3EDTA tubes (cat. no. 454021, Greiner Bio One Ltd.) and allowed to stand for 20 min. RNA was then extracted using TRIzol reagent (cat. no. T9424, MilliporeSigma) by the addition of 200 µl blood and 400 µl TRIzol to reagent to each sample. In addition, blood plasma (serum) was collected from all samples for the MDA assay.
RNA isolation and cDNA preparation
Total RNA was extracted from whole blood of patients with breast cancer and healthy women using the mirVana™ miRNA Isolation kit (AM1560, Thermo Fisher Scientific, Inc.), following the manufacturer's protocol. The quantity of miRNA was measured using a Qubit 4 fluorometer (Thermo Fisher Scientific, Inc.). This assay is highly selective for the miRNA quantification of other types of RNA. Following RNA extraction, cDNA was synthesized from isolated miRNA, through optimized primers, using a Protoscript cDNA synthesis kit (E6300L, New England BioLabs, Inc.). Briefly, 5 µl of each RNA sample were added to the Protoscript reaction mix, containing dNTPs, 10 µl buffer, 2 µl MuLV enzyme and 2 µl specific primers for each sample. All mixtures were incubated for 1 h at 42˚C in a thermocycler, followed by an incubation at 80˚C to inactivate the enzyme. The cDNA products were quantified using a Qubit 4 fluorometer. The products were electrophoresed on a 2% agarose gel and visualized on a UV transilluminator by ethidium bromide staining.
Reverse transcription-quantitative PCR (RT-qPCR)
RT-qPCR was used to detect the expression of miR-146 using the Luna® Universal qPCR Master Mix kit (cat. no. M3003, New England BioLabs, Inc.). Primers for miR-146 and U6 calibrators were designed by Macrogen, Inc. (Korea), and are presented in Table II.
Synthesized cDNA from patients and healthy controls were run simultaneously, including the target miR-146, and the housekeeping gene, U6 snRNA. The reaction mix consisted of 10 µl Luna Universal qPCR Master Mix, 1 µl forward primer (10 µM), 1 µl reverser primer (10 µM), 5 µl template DNA and 3 µl nuclease-free water. The qPCR program was set up with the indicated thermocycling protocol, as presented in Table III. Relative mRNA quantification was performed using the 2-ΔΔCq method (33).
MDA assay
MDA, a highly reactive an organic compound that causes toxic stress in cells, is a naturally occurring marker of oxidative stress (23). In the present study, to determine whether the MDA capacity of serum differs between healthy women and patients with breast cancer, the serum MDA concentrations were detected and its association with miR-146 gene expression was examined.
The MDA assay kit (cat. no. ab118970; Abcam) was used in the present study. Serum samples from 30 patients with breast cancer and 20 healthy women were collected and centrifuged at 2,500 x g for 10 min at room temperature to remove any residual cells. Subsequently, 200 µl of each serum sample or standard solution were mixed with 600 µl thiobarbituric Acid (TBA). All of the samples were then heated in a 100˚C water bath for 30 min and cooled down to room temperature 25˚C for 20 min. The total antioxidant capacity (TAC) was measured using a relevant kit (cat. no. ab65329; Abcam); 100 µl Cu2+ working solution was added to 100 µl of each sample or standard solution, followed by centrifugation at 1,008 x g for 10 min. Finally, the absorbance of the samples was measured using an Accuris™ SmartReader™ 96 microplate absorbance reader (cat. no. Z742712; Merck KGaA) at 532 and 570 nm, respectively.
Statistical analysis
Comparisons of miRNA gene expression frequencies and the results of the MAD assay among the study groups were determined using an unpaired t-test (independent samples t-test). All measurements were taken from three replicates and are presented as mean values, from which the standard error (SE) of the mean was calculated. A value of P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of U6 gene
The results of the expression of the U6 gene (endogenous control) in healthy women and patients with breast cancer are presented in Table IV. The mean Ct values for healthy women and patients with breast cancer were 20.71 and 20.59, respectively. There were no significant differences in U6 expression between the healthy and breast cancer samples.
The 2-ΔΔCq values for healthy women and patients with breast cancer were (0.64E-8) and (0.60E-8), respectively. There was no significant difference in U6 gene expression between the two groups. However, there was a decrease in fold change in patients with breast cancer, when compared with the healthy controls (0.93 and 1, respectively), as shown in Table V.
Expression of the miR-146 gene
The values of Ct, ΔCq and 2-ΔCq of the miR-146 gene in healthy women and breast cancer patients are presented in Table VI. The Ct values of the miR-146 gene for healthy women and patients with breast cancer were 29.76 and 28.04, respectively. The ΔCq values of the miR-146 gene were significantly (P<0.05) lower in patients with breast cancer compared with healthy women (7.47 and 9.1, respectively). In contrast, the 2-ΔCq values of miR-146 were significantly (P<0.05) higher in breast cancer patients than in healthy women (0.0056 and 0.0018, respectively). The results related the fold change in miR-146 gene expression, based on the 2-ΔCq and 2-ΔΔCq values, as presented in Table VI.
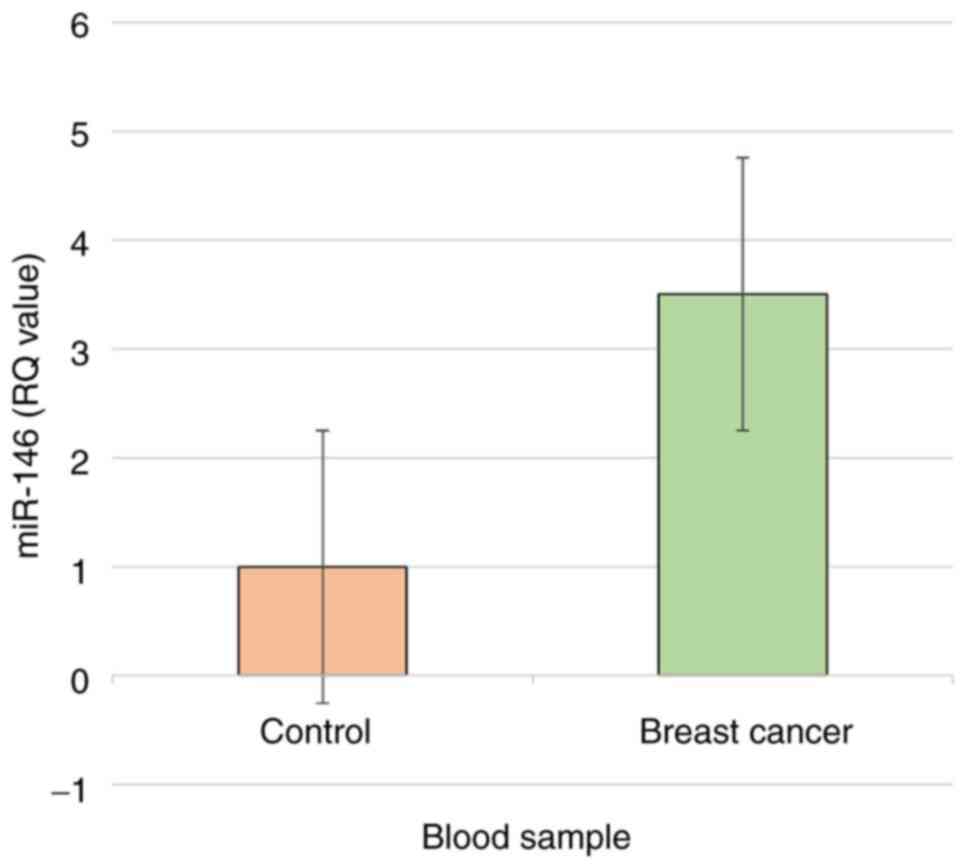
As shown in Table VII, depending on the 2-ΔCq method, the actual results were significantly associated with the predictions by the model (P<0.01); miR-146 expression was higher in patients with breast cancer compared with healthy women (3.1- and 1-fold, respectively). In addition, depending on the 2-ΔΔCq method, the values of ΔΔCq were significantly (P<0.01) lower in patients with breast cancer than in the healthy subjects (-1.52 and 0.11, respectively). The value of 2-ΔΔCq was significantly (P<0.01) higher in patients with breast cancer than in healthy women (2.867 and 0.926, respectively). Therefore, depending on the 2-ΔΔCq method, miR-146 gene expression was significantly (P<0.01) higher in patients with breast cancer than in healthy women (3.096- and 1-fold, respectively) (Fig. 1).
MDA levels in serum
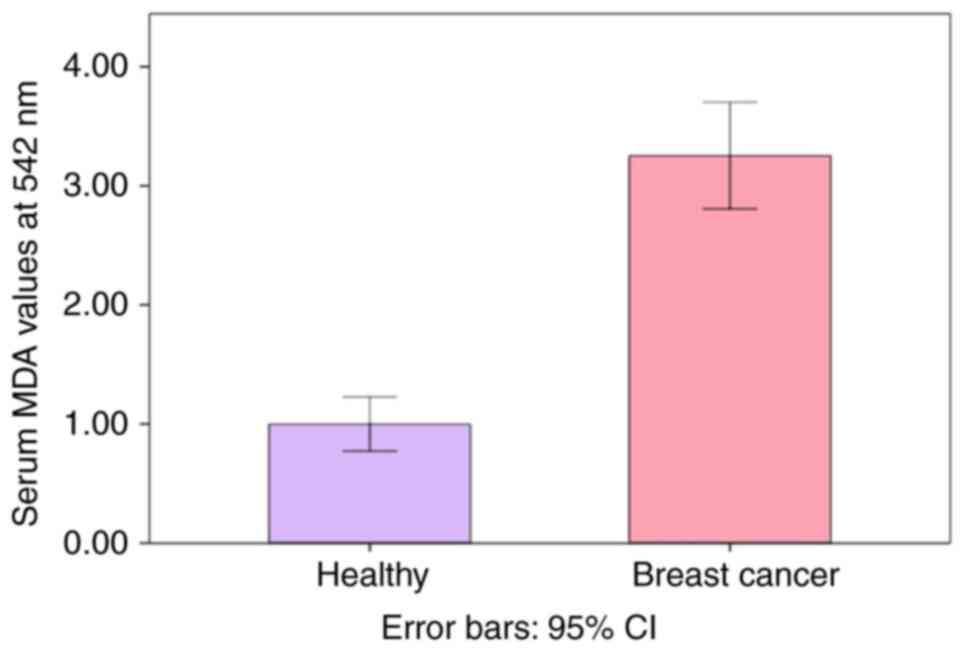
The serum MDA values in patients with breast cancer (n=30) and the healthy controls are presented in Table VIII. The MDA levels were significantly (P<0.01) higher in patients with breast cancer compared with the healthy controls (Fig. 2).
Association between miR-146 expression and MDA levels
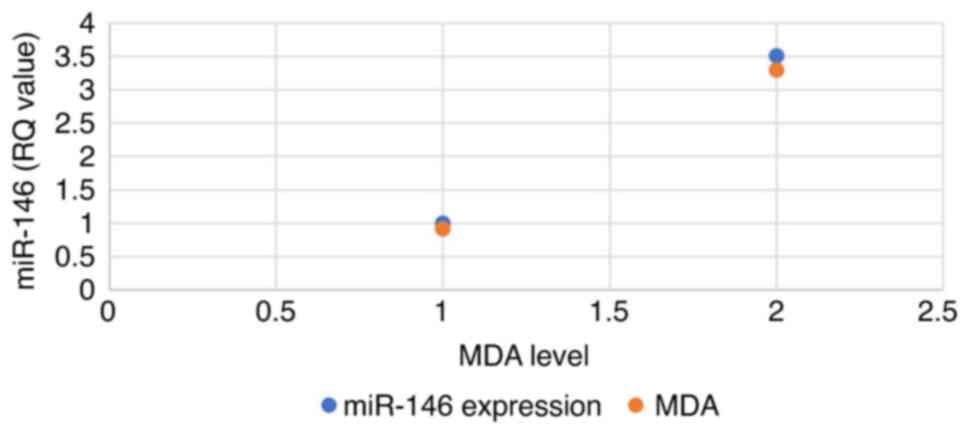
A positive association was found between miR-146 expression and MDA levels in patient serum, where miR-146 expression in normal healthy samples was 0.84, whereas the MDA level was 0.76. By contrast, the breast cancer samples exhibited a 3-fold increase in miR-146 expression and the MDA levels also exhibited a similar 2-fold increase (Fig. 3).
Discussion
A small, non-coding, single-stranded RNA that consists of 20-24 nucleotides, known as miRNA, plays a crucial role in gene transcription and expression by regulating gene expression (34). While miRNAs do not code for proteins, they are capable of directly degrading mRNA or preventing mRNA translation by creating complete or incomplete complementary combinations with the target mRNA (35).
A variety of studies have demonstrated that miRNAs are involved in tumor growth, metastasis and angiogenesis through the modulation of oncogenesis, migration and other related genes (36,37). Several miRNAs have also been shown to be associated with the clinical and pathological aspects of breast cancer, including the expression of estrogen and progesterone receptors, as well as vascular invasion. For example, Blenkiron et al (37) discovered 133 miRNAs expressed in both normal breast and cancer tissues; some of these types are associated with the molecular subtypes of breast cancer.
The aim of the present study was to elucidate the role of miRNA-146 in the context of oxidative stress in breast cancer. miRNAs are increasingly being implicated in the development of breast cancer. It has been found that miR-146a-5p expression is considerably higher in breast cancer tissues than it is in paraneoplastic tissue (21). The study by Gao et al (21) also reported that the expression of miR-146a-5p was significantly higher in MCF-7 cells than in control cells, as verified using RT-qPCR. MCF-7 cells with a high expression of miR-146a-5p exhibited an increased proliferation, while cells with a low expression exhibited a decreased proliferation (21). This finding supports the findings of the present study, indicating high levels of miR-146 expression in patients with breast cancer.
For a more in-depth understanding of oxidative stress, the present study measured the MDA levels in patients with breast cancer and compare these to those of healthy women. Increased levels of MDA have been reported in breast, ovary, gastric and lung cancers, as well as in colorectal adenomas (38-43). The reaction between polyunsaturated fatty acids and free radicals can produce MDA, a low-molecular-weight aldehyde. Patients with breast cancer have been found to have higher plasma levels of MDA (40). It was demonstrated that the MDA serum levels were indeed higher in patients with breast cancer than in healthy individuals (40). The study performed by Bhattacharjee et al (44) revealed that the median MDA level for patients with breast cancer was 3.98±0.35 nmol/ml, which was higher than that in controls (3.04±0.36 nmol/ml), with a P-value of 0.001. In the Ropanasuri Specialized Surgery Hospital, Padang, Indonesia, breast cancer patients and controls also exhibited significantly different MDA levels (44). Furthermore, patients with breast cancer had significantly higher serum MDA levels than those with benign breast diseases (P=0.042). The MDA concentrations and age of the patients with breast cancer and lymph node metastases differed significantly (P=0.006) (45). Similarly, Sahu et al also observed an increased level of MDA in patients with breast cancer, with an average value of 5.8±3.2 nmol/ml as compared to the control group with an average value of 1.9±0.28 nmol/ml, indicating that there were statistically significant differences between the two groups (38).
The results support the hypothesis that MDA plays a causal role in the development of breast cancer. In addition, malignant tissues contain higher MDA concentrations than normal tissue samples obtained from healthy individuals (46). The abnormally high levels of MDA in breast cancer patients can be attributed to excessive reactive oxygen species reactive oxygen species (ROS) production and a lack of antioxidant defenses.
The observed increase in MDA levels may be caused by ROS induction in breast cancer cells leading to oxidative stress and molecular damage, including lipid peroxidation (47). It is possible that MDA represents a product of lipid peroxidation, induced by an increase in ROS in the body, a process that could lead to the development of breast cancer (48,49). Biological, chemical and physical carcinogens can induce excessive ROS production. Significantly higher levels of oxidative stress and lower levels of antioxidants are associated with increased MDA levels in cancer patients. This event plays a critical role in the development and pathogenesis of tumors (50).
Since MDA is one of the most common products of lipid peroxidation, by interacting with proteins and DNA, it can lead to gene mutations that increase tumor development, explaining why increasing MDA levels can act as a marker cancer cell development (46,47). Previous studies have provided evidence that ROS plays a critical role in the development and progression of breast cancer (51). As a result, previous findings (52), as well as the results of the present study support the hypothesis that oxidative stress is prevalent, not only in cancer cells, but also throughout the entire body affected in cancer patients. In addition, MDA levels increased with progressing TNM stages in malignant breast cancer tissue (46).
Cancer progression and treatment resistance are characterized by ROS accumulation, altered redox balance and signaling. Oxidative phosphorylation generates ROS, primarily at the mitochondrial level. It is possible that the increased ROS levels detected in cancer cells arise due to several factors, including high metabolic activity, cellular signaling, peroxisomal activity, mitochondrial dysfunction, oncogene activity and increased enzyme activity of oxidases, cyclooxygenases, lipoxygenases, and thymidine phosphorylases (53). A number of antioxidants are involved in maintaining intracellular homeostasis, including catalase, superoxide dismutase and glutathione peroxidase. Furthermore, glutathione, a potent antioxidant, and the transcription factor, Nrf2, also contribute to balancing oxidative stress (53). Free radicals, oxidative stress and lipid peroxidation have been well documented as factors contributing to the carcinogenesis initiation and the progression of the process (39). It has been demonstrated that MDA is a potent marker for evaluating oxidative stress in patients with breast cancer. An individual's age and disease stage determine the level of oxidative stress (39). The levels of MDA can serve as a marker of an oxidative state. The disease stage and age have been shown to be associated with higher levels of malondialdehyde, suggesting a more severe state of oxidative stress (39).
A recent study demonstrated that miR-146a regulates inflammatory reactions in diseases associated with inflammation and oxidative stress (54). The association between miRNA-146a expression and MDA levels were examined in the present study. NF-κB is a transcription factor located upstream of the miR-146a promoter that triggers miR-146a expression in response to pro-inflammatory factors and reactive oxygen species. In turn, miR-146a can impede NF-κB and mediate inflammatory processes by inhibiting the expression of some of its target genes, such as IRAK1 and TRAF6 (55,56). Further analysis revealed that miR-146a overexpression inhibited neuronal apoptosis, reduced the production of pro-inflammatory cytokines, and reduced oxidative stress in ICH mice. miR-146a appears to function as a protective factor against ICH by inhibiting inflammatory and oxidative stress (57).
miR-146a levels have recently been found to be negatively associated with chronic inflammation and oxidative stress. In 2018, Xie et al (58) found that chronic type 2 diabetes (cT2DM) rats with elevated inflammation and oxidative stress status exhibited neurodegenerative disorders that were negatively correlated with miR-146a levels. miR-146a may therefore serve as a positive indicator of inflammation and oxidative stress in the brain of rats with chronic type 2 diabetes. Overall, it has been demonstrated that increased levels of inflammation and oxidative stress in cT2DM rats contribute to brain impairment, which is negatively regulated by miR-146a (58). Furthermore, inflammatory mediators, such as COX-2, TNF-α and IL-1β, as well as oxidative stress indicators, such as MDA and p22phox were elevated in the brain tissues of cT2DM rats and negatively correlated with miR-146a expression (58). Accordingly, the present study examined the association between miR-146a expression and MDA, an oxidative stress indicator, comparing patients with breast cancer and healthy women. However, no such association was observed. However, one of the limitations of the present study is the lack of sampling that affected the sample size. In addition, due to the difficulty of the survey, data on smoking, alcohol consumption and body mass index were not included.
miRNAs regulate a wide range of biological processes within a cell. Cancer is one of the diseases associated with miRNAs There has been a rapid increase in interest in miR-146a in particular, as a modulator of differentiation and function, as well as innate and adaptive immunity (19). Various types of tumors have a dysregulated expression of miR-146a due to the fact that miR-146a regulates several important cellular functions (19). Furthermore, to evaluate the effectiveness of free and total MDA as indicators of oxidative stress, a more in-depth understanding of the association between free and total MDA in different biological media is essential (59).
The mechanisms underlying this observation are not yet entirely clear; however, miRNAs may influence gene abundance via several different mechanisms. The mechanism underlying this phenomenon needs to be examined in more detail in future studies. Similarly, high levels of 8-hydroxydeoxyguanosine and MDA, and low superoxide dismutase levels have been shown to increase oxygen radical activity in certain inflammatory diseases (60). Thus, further studies are warranted to determine the association of miRNAs with breast cancer in more detail.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Authors' contributions
ASKAK made substantial contributions to the conception and design of the study; the acquisition, analysis and interpretation of the data; generated the datasets; and drafted the work. IMH collected blood samples from the primary breast cancer patients and healthy individuals, sufficiently participated in the acquisition, analysis and interpretation of the data, generated the datasets, and revised the manuscript. MAAN collected blood samples from primary breast cancer patients and healthy individuals, performed their laboratory analyses, contributed to the acquisition, analysis and interpretation of the data, and revised the manuscript. GOA contributed to the acquisition of data, performed the data analysis and interpretation, and revised the manuscript. ASKAK and GOA confirm the authenticity of all the raw data. All the authors have read and approved the final version of the manuscript for publication.
Ethics approval and consent to participate
Ethical approval for the present study was obtained from the Research Ethics Committee at the University of Baghdad under the reference no. NCRCEC/01/001. All clinical samples were collected autonomously from individuals who provided their informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.PubMed/NCBI View Article : Google Scholar | |
|
International Agency for Research on Cancer: Globocan 2012. World Health Organization, International Agency for Research on Cancer, Lyon, 2013. | |
|
Anothaisintawee T, Wiratkapun C, Lerdsitthichai P, Kasamesup V, Wongwaisayawan S, Srinakarin J, Hirunpat S, Woodtichartpreecha P, Boonlikit S, Teerawattananon Y and Thakkinstian A: Risk factors of breast cancer: A systematic review and meta-analysis. Asia Pac J Public Health. 25:368–387. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Shoukry M, Broccard S, Kaplan J and Gabriel E: The emerging role of circulating tumor DNA in the management of breast cancer. Cancers (Basel). 13(3813)2021.PubMed/NCBI View Article : Google Scholar | |
|
Feng Y, Spezia M, Huang S, Yuan C, Zeng Z, Zhang L, Ji X, Liu W, Huang B, Luo W, et al: Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 5:77–106. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Butti R, Gunasekaran VP, Kumar TV, Banerjee P and Kundu GC: Breast cancer stem cells: Biology and therapeutic implications. Int J Biochem Cell Biol. 107:38–52. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Liu S, Cong Y, Wang D, Sun Y, Deng L, Liu Y, Martin-Trevino R, Shang L, McDermott SP, Landis MD, et al: Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Reports. 2:78–91. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Bao L, Cardiff RD, Steinbach P, Messer KS and Ellies LG: Multipotent luminal mammary cancer stem cells model tumor heterogeneity. Breast Cancer Res. 17(137)2015.PubMed/NCBI View Article : Google Scholar | |
|
Sin WC and Lim CL: Breast cancer stem cells-from origins to targeted therapy. Stem Cell Investig. 4(96)2017.PubMed/NCBI View Article : Google Scholar | |
|
Lagadec C, Vlashi E, Della Donna L, Dekmezian C and Pajonk F: Radiation-induced reprogramming of breast cancer cells. Stem Cells. 30:833–844. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Chaffer CL, Marjanovic ND, Lee T, Bell G, Kleer CG, Reinhardt F, D'Alessio AC, Young RA and Weinberg RA: Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell. 154:61–74. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Koren S, Reavie L, Couto JP, De Silva D, Stadler MB, Roloff T, Britschgi A, Eichlisberger T, Kohler H, Aina O, et al: PIK3CAH1047R induces multipotency and multi-lineage mammary tumours. Nature. 525:114–118. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Colditz GA, Kaphingst KA, Hankinson SE and Rosner B: Family history and risk of breast cancer: Nurses' health study. Breast Cancer Res Treat. 133:1097–1104. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Allison KH: Molecular pathology of breast cancer: What a pathologist needs to know. Am J Clin Pathol. 138:770–780. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Dykes IM and Emanueli C: Transcriptional and post-transcriptional gene regulation by long non-coding RNA. Genomics Proteomics Bioinformatics. 15:177–186. 2017.PubMed/NCBI View Article : Google Scholar | |
|
O'Rourke JR, Swanson MS and Harfe BD: MicroRNAs in mammalian development and tumorigenesis. Birth Defects Res C Embryo Today. 78:172–179. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Saito Y, Nakaoka T and Saito H: microRNA-34a as a therapeutic agent against human cancer. J Clin Med. 4:1951–1959. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Tordonato C, Di Fiore PP and Nicassio F: The role of non-coding RNAs in the regulation of stem cells and progenitors in the normal mammary gland and in breast tumors. Front Genet. 6(72)2015.PubMed/NCBI View Article : Google Scholar | |
|
Rusca N and Monticelli S: MiR-146a in immunity and disease. Mol Biol Int. 2011(437301)2011.PubMed/NCBI View Article : Google Scholar | |
|
Tordonato C, Marzi MJ, Giangreco G, Freddi S, Bonetti P, Tosoni D, Di Fiore PP and Nicassio F: miR-146 connects stem cell identity with metabolism and pharmacological resistance in breast cancer. J Cell Biol. 220(e202009053)2021.PubMed/NCBI View Article : Google Scholar | |
|
Gao W, Hua J, Jia Z, Ding J, Han Z, Dong Y, Lin Q and Yao Y: Expression of miR 146a 5p in breast cancer and its role in proliferation of breast cancer cells. Oncol Lett. 15:9884–9888. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Chen J, Jiang Q, Jiang XQ, Li DQ, Jiang XC, Wu XB and Cao YL: miR-146a promoted breast cancer proliferation and invasion by regulating NM23-H1. J Biochem. 167:41–48. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Del Rio D, Stewart AJ and Pellegrini N: A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 15:316–328. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Nair V, O'Neil CL and Wang PG: Malondialdehyde. In: Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons, Hoboken, NJ, 2001. | |
|
Mateos R, Goya L and Bravo L: Determination of malondialdehyde by liquid chromatography as the 2, 4-dinitrophenylhydrazone derivative: A marker for oxidative stress in cell cultures of human hepatoma HepG2. J Chromatogr B Analyt Technol Biomed Life Sci. 805:33–39. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Al-Khafaji ASK, Pantazi P, Acha-Sagredo A, Schache A, Risk JM, Shaw RJ and Liloglou T: Overexpression of HURP mRNA in head and neck carcinoma and association with in vitro response to vinorelbine. Oncol Lett. 19:2502–2507. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Al-Khafaji AS, Davies MP, Risk JM, Marcus MW, Koffa M, Gosney JR, Shaw RJ, Field JK and Liloglou T: Aurora B expression modulates paclitaxel response in non-small cell lung cancer. Br J Cancer. 116:592–599. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Al-Khafaji ASK, Marcus MW, Davies MPA, Risk JM, Shaw RJ, Field JK and Liloglou T: AURKA mRNA expression is an independent predictor of poor prognosis in patients with non-small cell lung cancer. Oncol Lett. 13:4463–4468. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Tarannum J, Manaswini P, Deekshitha C, Reddy BP and Sunder AS: Elucidative Histopathological study in female cancer patients: Histopathology in female cancers. Iraq J Sci. 61:720–726. 2020. | |
|
Lei B, Liu J, Yao Z, Xiao Y, Zhang X, Zhang Y and Xu J: NF-κB-Induced Upregulation of miR-146a-5p promoted hippocampal neuronal oxidative stress and pyroptosis via TIGAR in a Model of Alzheimer's Disease. Front Cell Neurosci. 15(653881)2021.PubMed/NCBI View Article : Google Scholar | |
|
Jin X, Liu J, Chen YP, Xiang Z, Ding JX and Li YM: Effect of miR-146 targeted HDMCP up-regulation in the pathogenesis of nonalcoholic steatohepatitis. PLoS One. 12(e0174218)2017.PubMed/NCBI View Article : Google Scholar | |
|
Mao H and Xu G: Protective effect and mechanism of microRNA-146a on ankle fracture. Exp Ther Med. 20(3)2020.PubMed/NCBI View Article : Google Scholar | |
|
Livak KJ and Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Kagiya T: MicroRNAs: Potential biomarkers and therapeutic targets for alveolar bone loss in periodontal disease. Int J Mol Sci. 17(1317)2016.PubMed/NCBI View Article : Google Scholar | |
|
Jansson MD and Lund AH: MicroRNA and cancer. Mol Oncol. 6:590–610. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Ohtsuka M, Ling H, Doki Y, Mori M and Calin GA: MicroRNA processing and human cancer. J Clin Med. 4:1651–1667. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Blenkiron C, Goldstein LD, Thorne NP, Spiteri I, Chin SF, Dunning MJ, Barbosa-Morais NL, Teschendorff AE, Green AR, Ellis IO, et al: MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 8(R214)2007.PubMed/NCBI View Article : Google Scholar | |
|
Sahu A, Varma M and Kachhawa K: A prognostic study of MDA, SOD and catalase in breast cancer patients. Int J Sci Res. 4:157–159. 2015. | |
|
Didžiapetrienė J, Bublevič J, Smailytė G, Kazbarienė B and Stukas R: Significance of blood serum catalase activity and malondialdehyde level for survival prognosis of ovarian cancer patients. Medicina (Kaunas). 50:204–208. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Sadati Zarrini A, Moslemi D, Parsian H, Vessal M, Mosapour A and Shirkhani Kelagari Z: The status of antioxidants, malondialdehyde and some trace elements in serum of patients with breast cancer. Caspian J Intern Med. 7:31–36. 2016.PubMed/NCBI | |
|
Bakan E, Taysi S, Polat MF, Dalga S, Umudum Z, Bakan N and Gumus M: Nitric oxide levels and lipid peroxidation in plasma of patients with gastric cancer. Jpn J Clin Oncol. 32:162–166. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Gupta A, Srivastava S, Prasad R, Natu SM, Mittal B, Negi MP and Srivastava AN: Oxidative stress in non-small cell lung cancer patients after chemotherapy: Association with treatment response. Respirology. 15:349–356. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Tao D, Zhou Z, Xu X and Luo H: Lipid disorders and lipid peroxidation associated with the malignant transformation of colorectal adenoma. Chin Ger J Clin Oncol. 10:270–273. 2011. | |
|
Bhattacharjee J, Jogdand S, Shinde RK and Goswami S: Assessment of oxidative stress in breast cancer patients: A hospital based study. Int J Basic Clin Pharmacol. 7:966–970. 2018. | |
|
Gubaljevic J, Srabović N, Jevrić-Čaušević A, Softić A, Rifatbegović A, Mujanović-Mustedanagić J, Dautović E, Smajlović A and Mujagić Z: Serum levels of oxidative stress marker malondialdehyde in breast cancer patients in relation to pathohistological factors, estrogen receptors, menopausal status, and age. J Health Sci. 8:154–161. 2018. | |
|
Sener DE, Gönenç A, Akıncı M and Torun M: Lipid peroxidation and total antioxidant status in patients with breast cancer. Cell Biochem Funct. 25:377–382. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Qebesy HS, Zakhary MM, Abd-Alaziz MA, Abdel Ghany AA and Maximus DW: Tissue levels of oxidative stress markers and antioxidants in breast cancer patients in relation to tumor grade. Al-Azhar Assiut Med J. 13:10–17. 2015. | |
|
Hauck AK and Bernlohr DA: Oxidative stress and lipotoxicity. J Lipid Res. 57:1976–1986. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Gönenç A, Erten D, Aslan S, Akıncı M, Şimşek B and Torun M: Lipid peroxidation and antioxidant status in blood and tissue of malignant breast tumor and benign breast disease. Cell Biol Int. 30:376–380. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Kilic N, Yavuz Taslipinar M, Guney Y, Tekin E and Onuk E: An investigation into the serum thioredoxin, superoxide dismutase, malondialdehyde, and advanced oxidation protein products in patients with breast cancer. Ann Surg Oncol. 21:4139–4143. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Himmetoglu S, Dincer Y, Ersoy YE, Bayraktar B, Celik V and Akcay T: DNA oxidation and antioxidant status in breast cancer. J Investig Med. 57:720–723. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Mena S, Ortega A and Estrela JM: Oxidative stress in environmental-induced carcinogenesis. Mutat Res. 674:36–44. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Didžiapetrienė J, Kazbarienė B, Tikuišis R, Dulskas A, Dabkevičienė D, Lukosevičienė V, Kontrimavičiūtė E, Sužiedėlis K and Ostapenko V: Oxidant/antioxidant status of breast cancer patients in pre-and post-operative periods. Medicina (Kaunas). 56(70)2020.PubMed/NCBI View Article : Google Scholar | |
|
Wan RJ and Li YH: MicroRNA-146a/NAPDH oxidase4 decreases reactive oxygen species generation and inflammation in a diabetic nephropathy model. Mol Med Rep. 17:4759–4766. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Li K, Ching D, Luk FS and Raffai RL: Apolipoprotein E enhances microRNA-146a in monocytes and macrophages to suppress nuclear factor-κB-driven inflammation and atherosclerosis. Circ Res. 117:e1–e11. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Lo WY, Peng CT and Wang HJ: MicroRNA-146a-5p mediates high glucose-induced endothelial inflammation via targeting interleukin-1 receptor-associated kinase 1 expression. Front Physiol. 8(551)2017.PubMed/NCBI View Article : Google Scholar | |
|
Qu X, Wang N, Cheng W, Xue Y, Chen W and Qi M: MicroRNA-146a protects against intracerebral hemorrhage by inhibiting inflammation and oxidative stress. Exp Ther Med. 18:3920–3928. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Xie Y, Chu A, Feng Y, Chen L, Shao Y, Luo Q, Deng X, Wu M, Shi X and Chen Y: MicroRNA-146a: A comprehensive indicator of inflammation and oxidative stress status induced in the brain of chronic T2DM rats. Front Pharmacol. 9(478)2018.PubMed/NCBI View Article : Google Scholar | |
|
Cui X, Gong J, Han H, He L, Teng Y, Tetley T, Sinharay R, Chung KF, Islam T, Gilliland F and Grady S: Relationship between free and total malondialdehyde, a well-established marker of oxidative stress, in various types of human biospecimens. J Thorac Dis. 10:3088–3097. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Canakci CF, Cicek Y, Yildirim A, Sezer U and Canakci V: Increased levels of 8-hydroxydeoxyguanosine and malondialdehyde and its relationship with antioxidant enzymes in saliva of periodontitis patients. Eur J Dent. 3:100–106. 2009.PubMed/NCBI |