Epstein‑Barr virus as a promoter of tumorigenesis in the tumor microenvironment of breast cancer (Review)
- This article is part of the special Issue: Significance of molecular analyses in the era of personalized tumor therapy
- Authors:
- Published online on: July 6, 2023 https://doi.org/10.3892/ijmm.2023.5275
- Article Number: 72
-
Copyright: © Gómez-Archila et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
1. Introduction
Cancers attributable to infection have a higher incidence than any single type of cancer worldwide (1). In total, 11 pathogens have been classified as human carcinogens by the International Agency for Research on Cancer (IARC). Infectious causes related to the development of cancer, with the exception of Helicobacter pylori, which is associated with 770,000 cases worldwide, are caused by viruses; these are namely human papillomavirus (HPV) with 640,000 cases, hepatitis B virus with 420,000 cases, hepatitis C virus with 170,000 cases and Epstein-Barr virus (EBV) with 120,000 cases (2-6). EBV was found in samples from patients with breast cancer (BC) in the 1990s, followed by attempts to elucidate the possible role of EBV in this neoplasia, and an increase in aggressiveness was proposed when related to the presence of EBV (7). Several mechanisms are affected by the expression of viral proteins with oncogenic activity, such as EBV nuclear antigens (EBNAs), which cause epigenetic alterations through histone acetylation and chromatin remodeling, contributing to immune system viral evasion (8). On the other hand, latent membrane protein (LMP)1 and LMP2A are associated with resistance to certain chemotherapeutic regimens through various epigenetic alterations, as well as with promoting changes at the mitochondrial level related to high rates of recurrence, since they promote cell migration and interfere with apoptosis (9). Moreover, it has been shown that long non-coding RNAs (lncRNAs) of the virus cause less sensitivity to radiation therapy and affect the process of metastasis (10,11).
According to the literature, the influence of the various components of EBV and its effects on BC are critical for the development and evolution of the disease, although it remains controversial since the behavior of the virus in various geographical areas and in various populations differs. Therefore, the aim of the present study was to unify and update the information by reviewing the literature and to help broaden the current understanding of the association of EBV with the evolution and development of BC.
2. Epstein-Barr virus and breast cancer
EBV is associated with epithelial malignancies, including nasopharyngeal cancer, gastric cancer and BC. The first two neoplasms have been extensively studied and linked to EBV, as aforementioned. However, in the case of BC, it is not entirely clear how EBV can influence the development of the disease, since in some reports (12,13) its participation is controversial.
The role played by various microRNAs (miRNAs/miRs), lncRNAs and EBV proteins in the process of the malignant transformation of epithelial cells, including the mammary epithelium, appears to be related to the viral components involved. The participation of the virus is not the same in different types of cancer, suggesting that there are other factors that influence progression, immortalization or immune evasion (14,15).
According to Shannon-Lowe et al (16), the association between epithelial cells and EBV infection is unclear, unlike the association between B-cells and EBV. However, due to the common histological features, some similarities between nasopharyngeal carcinoma, gastric cancer and BC would be expected. EBV binding to B-cells is efficient due to the viral protein gp350 (which interacts with the EBV receptor), cell surface CD21 or CR2. Subsequently, another viral glycoprotein known as gp42, interacts with the cellular human leukocyte antigen (HLA). However, as regard the binding of EBV to epithelial cells, the process differs, since these cells lack the expression of CD21, although the same gp350/CD21 interaction on B-lymphocytes can expose more ligands on the viral envelope, allowing EBV entry into epithelial cells (16).
Likewise, other routes or pathways in the epithelial cell infection process have been described, such as transfer mediated by B-cells (17-19), cell-to-cell contact infection (20,21) and the direct infection of epithelial cells expressing the viral receptor, Ephrin A2 (22,23).
It should be considered that EBV can infect epithelial cells more easily as it has initially replicated in B-lymphocytes. Shannon-Lowe and Rowe (18) confirmed the above by demonstrating that viral gp42 interacts with HLA class II in the endoplasmic reticulum of B-lymphocytes, causing the degradation of this viral protein. Thus, virions with little or no gp42 are not efficient at infecting B-lymphocytes; however, the infection of epithelial cells is not compromised as other routes of infection exist. In this manner, EBV that has replicated in epithelial cells, where there is no expression of HLA class II, has a high expression of gp42, which is why it is capable of efficiently infecting B-lymphocytes (18) (Fig. 1).
On the other hand, considering that the damage or modifications in the mammary epithelium are the initial part of the malignant process, other factors that influence it have been described. Examples of the above are the participation of other viruses that can contribute by causing the damage or infection of various epithelial cells (24,25), co-infections such as Helicobacter pylori, as well as the role of various EBV miRNAs and lncRNAs that influence epithelial-mesenchymal transition (EMT).
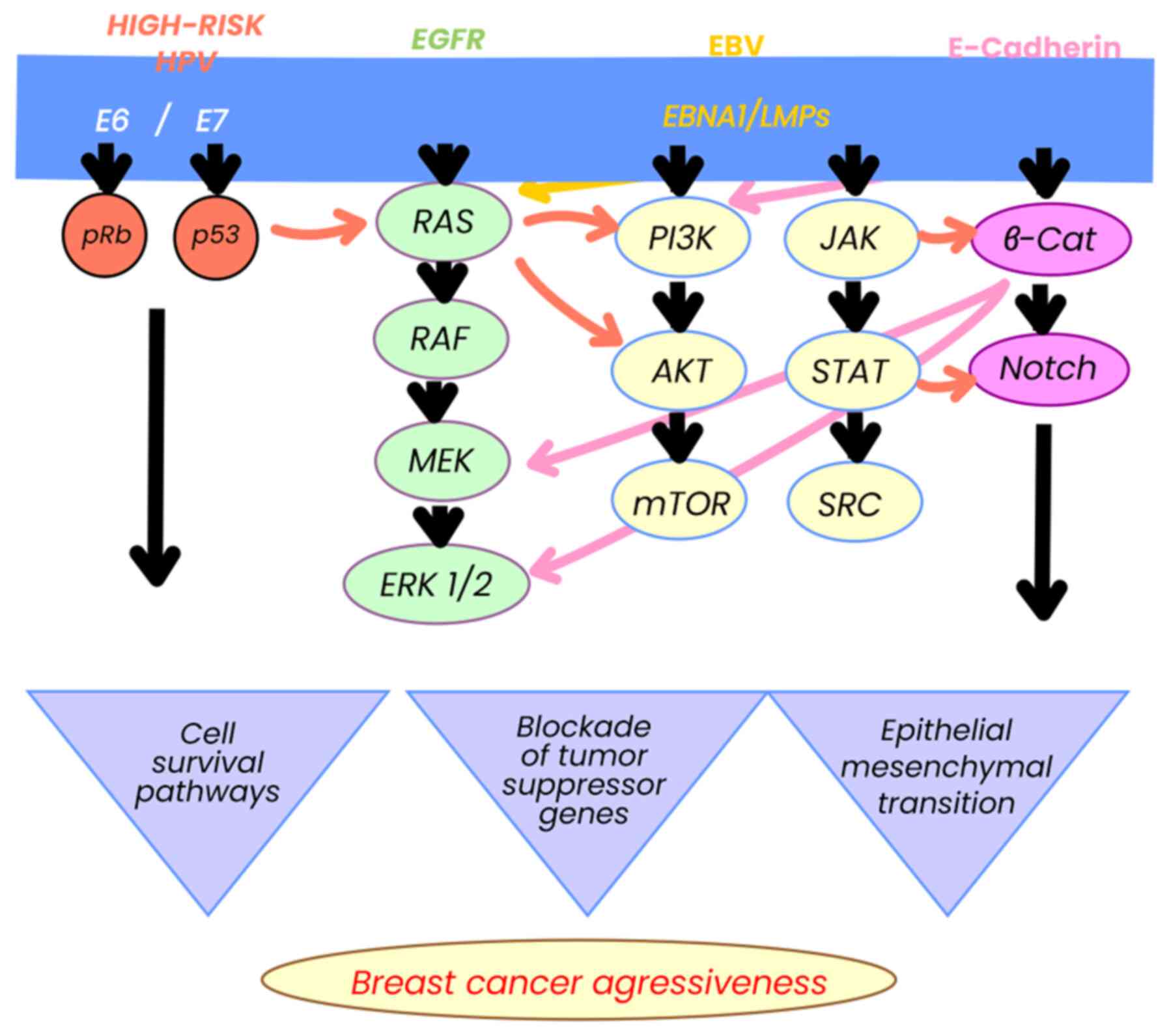
Considering the various causal factors of BC, there are several signaling pathways involved, such as the (human epidermal growth factor receptor (HER), NOTCH and β-catenin pathways, which are linked to the evolution of the disease, as demonstrated in in vitro studies (26-29) and in patient samples (30-32). In turn, these pathways are related to EBV components, promoting progression and cell survival and in some cases, resistance to anthracycline-based treatments. These pathways are critical, depending on the host characteristics, heredity and epigenetics, and may be determinants for the evolution of BC (15,33-35).
3. Role of Epstein-Barr virus infection in breast cancer and its association with ethnicity
Although, as aforementioned, EBV is present in >90% of the world's population (10), there are geographical areas where its prevalence is higher. As regards its distribution, the presence of EBV is predominant in countries of North Africa and Asia, when compared to Europe and North America (36).
According to Zanella et al (36) and others, EBV type I is the most prevalent, and it is mainly located in Europe, Asia, North and South America, and type II is more frequent in Alaska, Papua New Guinea and Central Africa, with a predominance in Kenya (36,37-39). In particular, Asian countries, such as Syria, Iran and Qatar have reported the presence of EBV associated with BC with poor responses to treatment, or with a more aggressive presentation of the disease (24,25,40-42). In the case of countries such as Eritrea (Northeast Africa), more aggressive cases of BC have also been reported and have been related to the presence of EBV, as described in the study by Fessahaye et al (43).
In other parts of Africa, this disease has been found to be associated with the presence of EBV. However, Nwagu et al (44) also reported more aggressive or poor prognosis phenotypes, such as triple-negative carcinomas (where different rates of mutations in BRCA1 and 2 are also described), which increases the risk of developing ovarian cancer. Countries such as Ghana and Senegal are an example (44).
In the case of America and Europe, despite having a high incidence of BC according to global statistics, it does not appear to be associated with EBV viral components. However, according to these reports, there is no doubt that the African and Asian populations are much more susceptible to the effects of EBV for the development and poor evolution of the disease (17).
Therefore, BC is an entity whose incidence is closely linked to ethnicity (45). Although it is not entirely clear, there are studies that have demonstrated that some molecules determine biochemical and physiological changes that lead the cell to tumorigenesis (32,46,47) Some polymorphisms related to ethnicity have been observed in BC, suggesting that they may be associated with it. For example, the C7T single nucleotide polymorphism can lead to the deregulation of the expression of miRNA196a, which can favor susceptibility to BC. This polymorphism was detected in an ethnic group of a Pakistani population (48). This miRNA was found deregulated in gastric adenocarcinoma during an EBV infection; thus, it is possible to associate the influence of EBV in other types of tumors in which polymorphisms that deregulate miRNAs are involved (49). Other miRNAs have also been found to be deregulated in BC, including hsa-miR-495, hsa-miR-592, hsa-miR-6501 and hsa-miR-937 (50); however, it has not yet been described whether EBV is involved in its deregulation, which cannot be ruled out. Therefore, it is necessary to continue studying possible associations.
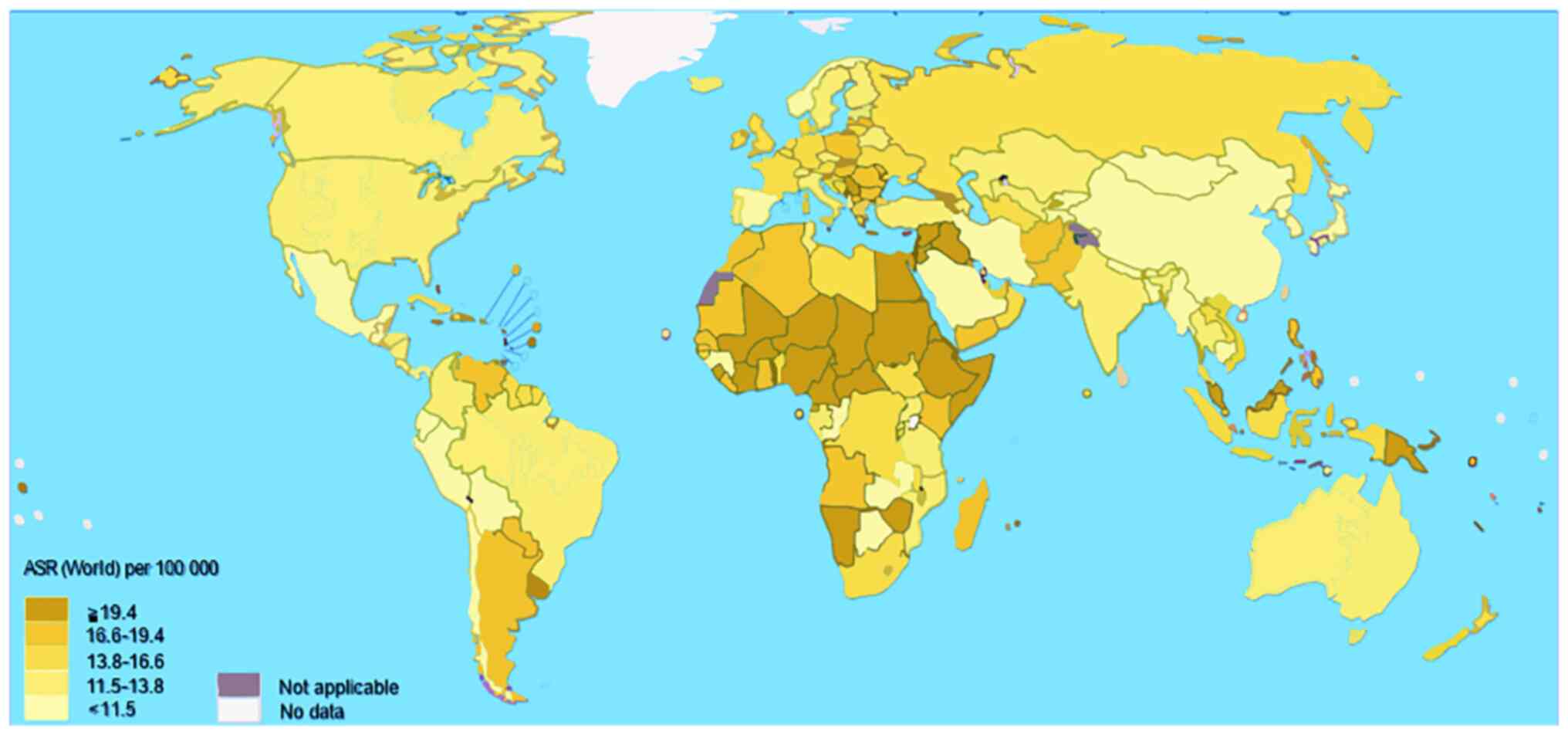
Paradoxically, the current status of BC with or without association with EBV in developed countries is the same as in undeveloped countries, being the number one cause of oncological morbidity and mortality in women, thus denoting the complexity and multifactorial nature that surrounds this entity (51). Although according to international statistics the difference between these countries is that the clinical stages of BC are diagnosed early in developed countries, while in undeveloped countries it occurs in advanced stages, which affects the prognosis of BC (52).
The above is reflected in Fig. 2, where, when evaluating BC mortality by country, Central Africa and some Asian countries stand out. It is noteworthy that these countries coincide with a high incidence of EBV and that it has been related to the evolution of BC in the reports mentioned above.
4. Role of Epstein-Barr virus nuclear antigen in breast cancer
EBV encodes six genes for nuclear antigens (EBNA1, 2, 3A, 3B, 3C and LP), which represent proteins with oncogenic activity involved in various cancers, including BC (53).
EBNA1 has epigenetic functions by competitively binding a specific ubiquitin protease (USP7), resulting in an unstable, ubiquitinated p53 with altered tumor suppressor functions (54). EBNA2 acetylates histones in co-participation with EBNA3C, which leads to the activation of the HATs p300, CBP and KAT2B/PCAF pathways, resulting in chromatin remodeling. In BC, EBNA3C can also bind to the non-metastatic clone 23 of nucleoside diphosphate kinase-H1 isoform (Nm23-H1) causing downregulation of this enzyme, thus promoting cancer cell survival and progression (55).
5. Latent membrane protein and breast cancer
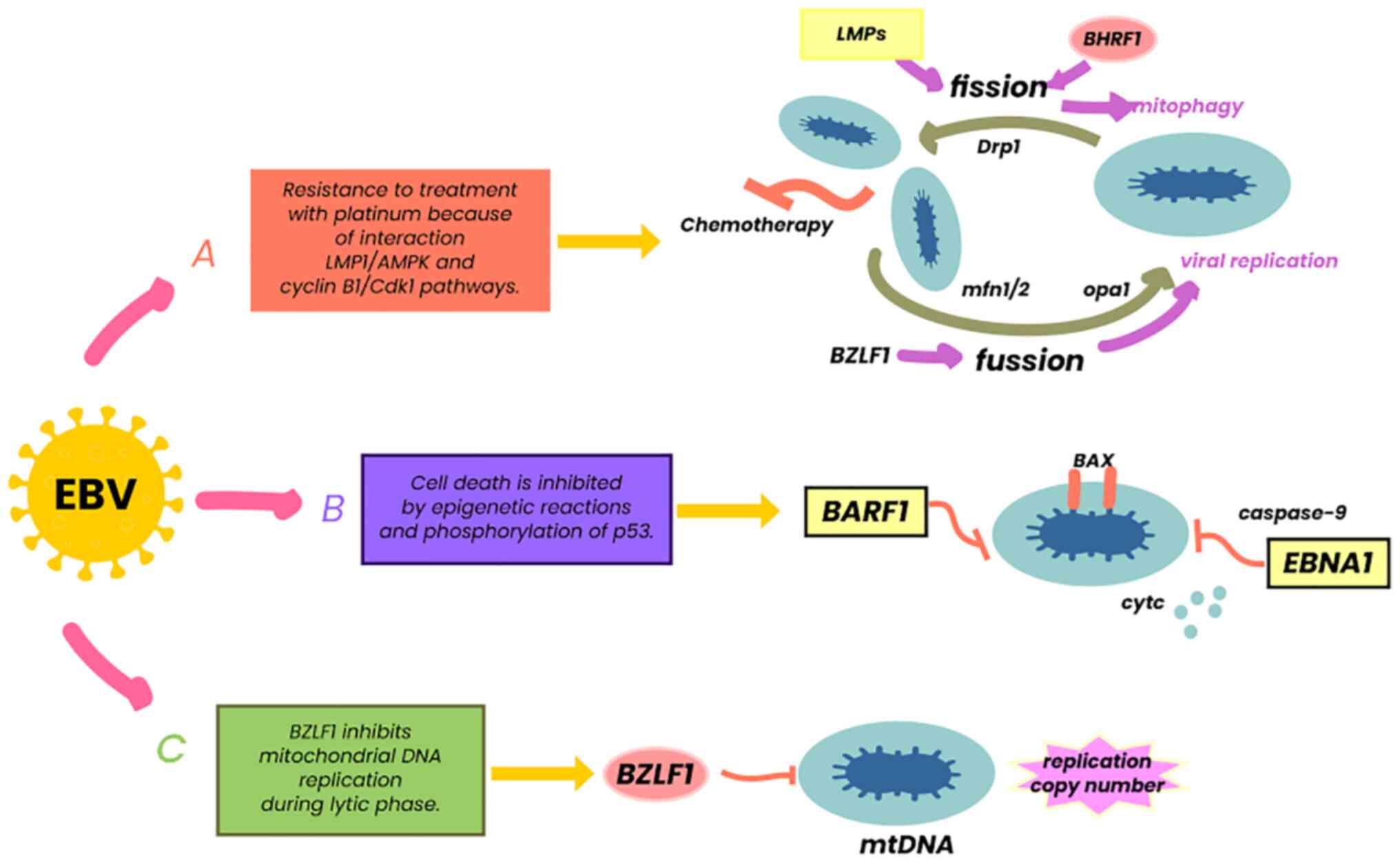
LMPs are the most notable from an oncological aspect (10). The LMP complex is comprised of a group of three genes (LMP1, 2A and 2B), which fulfill various functions in both the latent and lytic phases of EBV (56). LMP2A and LMP2B act as receptors for B-cells, modulating their activity, which favors the survival of EBV, avoiding the apoptosis of infected cells and thus evading the immune system (57). LMP1 mimics the function of CD40 through its carboxyl terminal residues (CTAR1 and CTAR2), activating intracellular pathways, such as NF-κB, JNK, p38 and JAK/AP-1/STAT, related to cell survival and proliferation (56). LMP1 can phosphorylate p53, preventing its tumor suppressor function (54), but also acting as a tumor necrosis factor (TNF) homologue in epithelial cells and B-lymphocytes (56). It has been described that LMP1 interacts with β1 integrin ligands through the anoikis pathway, promoting changes in epithelial cells and favoring the process of cell malignancy, a crucial mechanism in epithelial neoplastic processes, such as BC (58). Finally, this viral protein contributes to tumorigenesis by activating proteins associated with mitochondrial dynamics (Drp1), which in turn interact in pathways such as AMPK and cyclin β1/Cdk1, causing resistance to platinum, thereby reducing the control of entities managed with these drugs (59) (Fig. 3).
6. Epstein-Barr virus miRNAs, lncRNAs and Epstein-Barr virus-encoded RNAs in breast cancer
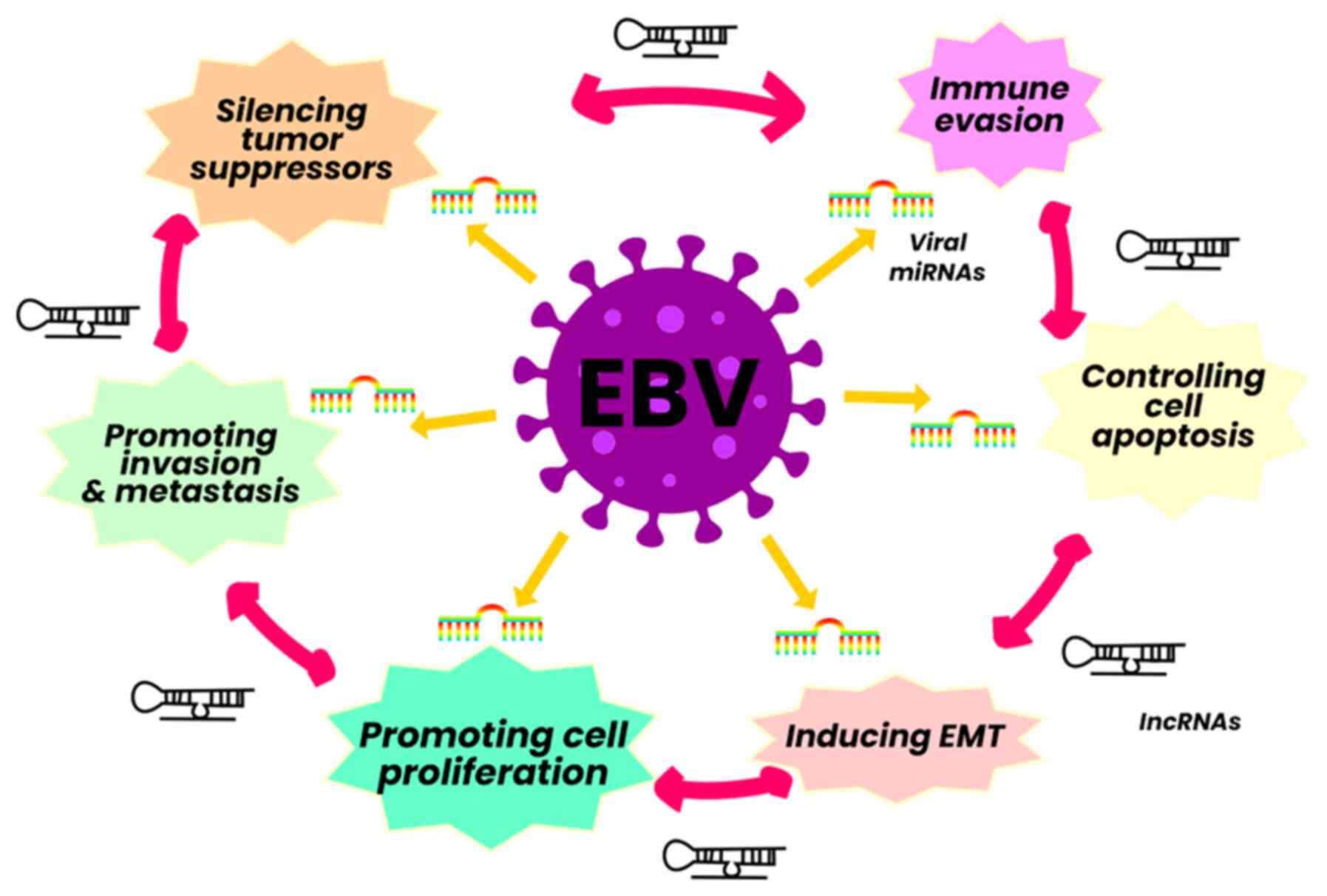
EBV codes for various miRNAs in two regions: BART and BHRF1. These are expressed in large quantities in EBV-infected malignant cells and are usually cell type specific (60). The miRNAs are recognized as participating in immune evasion and perpetuating latency phases. Recent studies have described a range of functions that directly influence various malignant processes; for example, miRNA-218 is a tumor suppressor downregulated in fresh tissue samples of BC and adjacent tissue, and it is associated with adverse clinical outcomes. On the other hand, miR-BART9 suppresses the expression of E cadherin and acts as a negative regulator of miR-200a expression, thus promoting the process of EMT (61).
The expression profile of EBV lncRNAs in its different phases is still not entirely clear. Some researchers have demonstrated that BART-lncRNAs and BHLF1-lncRNAs affect various cytokines and favor their immune evasion ability. BHLF1-lncRNAs influence viral replication, contribute to viral latency and immortalize B-cells (61). Additionally, host lncRNAs are regulated directly or indirectly in cells infected with EBV and participate in proliferation, invasion, metastasis and immune escape, such as lncRNA NAG7, CYTOR, NORAD, SNHG8, MINCR, lncRNA-BC200, LINC00672, MALAT1, LOC100128494, lncRNA RP4-794H19.1, LOC553103, TP73-AS1 and RP5-1039K5.19 (62) (Fig. 4).
The expression of EBV-encoded RNAs (EBERs) induces tumor growth in vitro or in vivo, blocks apoptosis through the RNA-dependent protein kinase pathway and cytokines, such as interleukin (IL)-9 and IL-10, favoring cell survival (63,64). They also participate in the activation of signaling pathways such as NF-κB and IRF-3 mediated by a retinoic acid inducible gene and the induction of IFN type I (63,65).
All the effects of EBERs on various pro-inflammatory pathways or molecules are related to the tumor environment and peritumoral tissue in BC, where the role of tumor-infiltrating lymphocytes, as well as various peritumoral proinflammatory components, may allow tumor progression.
Taking into consideration all the aforementioned information, the role of EBV-miRNAs, EBV-lncRNAs and EBERs in the various signaling pathways associated with the oncogenesis process, represents multiple possibilities for approaching the molecular biology of the tumor, in this case for BC. However, there are reports in which due to the effect of viral elements, such as LMP1, the host expresses miRNAs with effects on various platinum-based treatments, through the PI3K/AKT/FOXO3a pathways, such is the case of the expression of miRNA-21 that blocks the effect of this type of chemotherapy. This compound is used in nasopharyngeal carcinoma and triple-negative varieties of BC, suggesting a chemoresistance effect induced by miRNAs encoded by the EBV genome (66), with host miRNA-21 being one of the most prominent in this disease (67).
Therefore, the importance of the influence of viral components that promote resistance to platinum-based BC treatments lies in the fact that one of the molecular subtypes of this disease, triple-negative, uses this treatment alternative. In addition, these viral components as mentioned, can intervene in the AKT/PI3K pathways, indirectly altering the response not only those based on platinum, but also those aimed at blocking these pathways. These drugs are currently among the most innovative in BC for varieties with the expression of positive hormone receptors and negative HER2 neu (68,69), denoting again possible causes of chemoresistance attributed to EBV.
7. Co-infections
There is evidence that the simultaneous expression of viral proteins and inflammatory factors could modify the behavior of the disease in viruses such as Human Papilloma Virus (HPV) in BC (70-72). EBV has been described in various neoplasms in co-infection with other viruses, mainly with HPV (1,8,73,74). Some studies have linked EBV-HPV co-infection with benign and malignant breast tumors and thyroid cancer, denoting the role of viral proteins such as E2, E6 and E7 for HPV as well as LMP1 and LMP2A for EBV (70). This suggests that in co-infection, the viral protein expression of both entities, contribute simultaneously to the progression of these neoplasms. On the other hand, it has been described that Asian and African populations have higher incidences of EBV associated with BC, but are also carriers of EBV-PHV co-infections (75). EBV-HPV co-infection in BC, appears to be associated to more aggressive varieties, such as of triple-negative tumors (41,76) (Fig. 5).
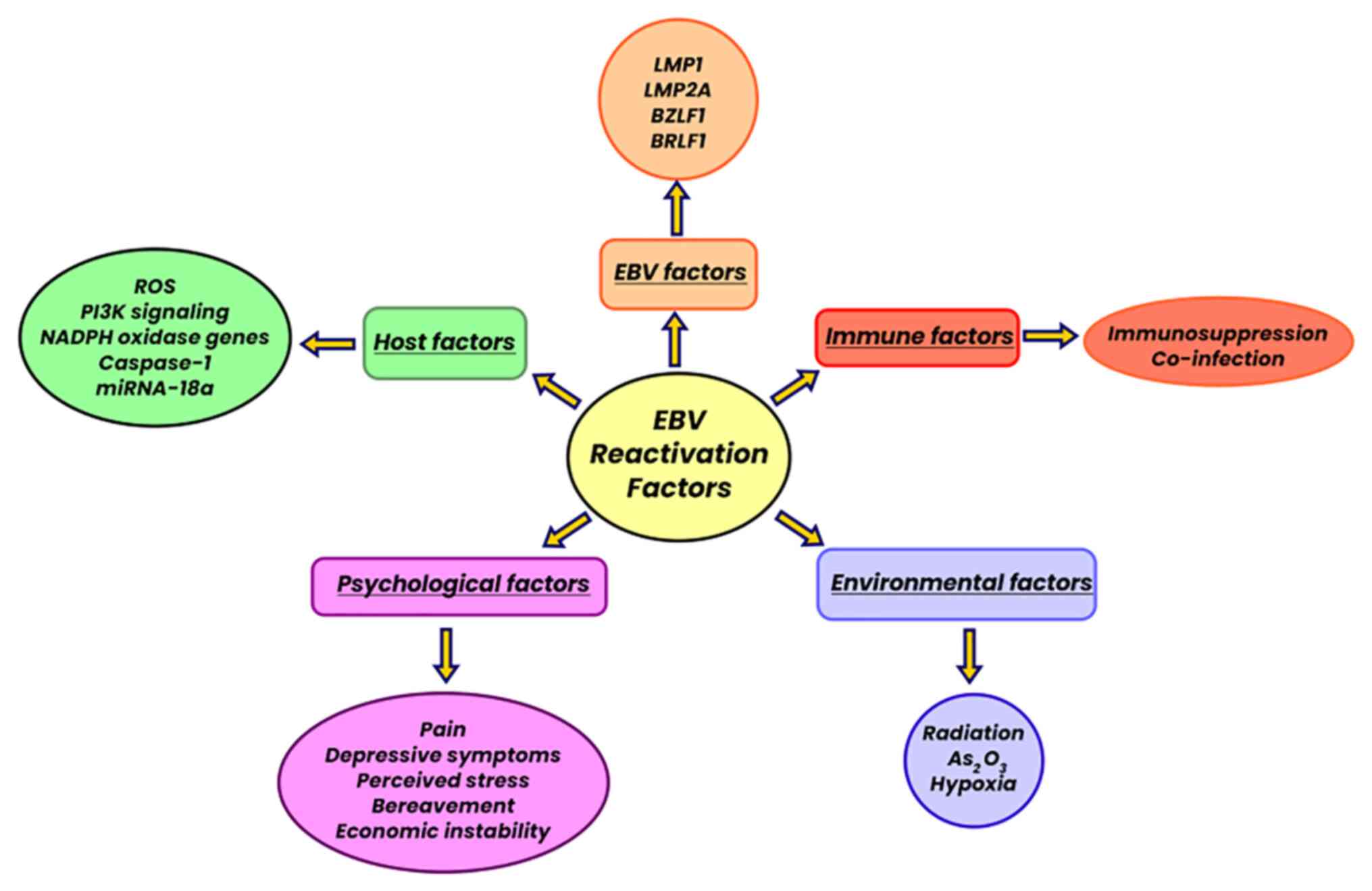
8. Epstein-Barr virus reactivation
Since EBV is not cleared from the body after primary infection, it can remain dormant in various tissues and cells such as B lymphocytes, and can be reactivated in certain situations.
The reactivation or lytic phase of EBV is multifactorial and does not occur in the same manner in all cases, and it is dependent on the proper functioning of the host's immune system and on the external stimuli it is exposed to. During the viral reactivation process, EBV encodes two early genes, BZLF1 (Z, Zta, ZEBRA or EB1) and BRLF1 (R or Rta), which are suppressed in the latent phase. Both genes are required for reactivation as the lytic cycle is blocked by either gene inactivation, although BZLF1 is considered the master regulator (77). In some cases, there may be BZLF1 or BRLF1 exogenous overexpression in latently infected cells, which also initiates the process of viral reactivation.
Cell culture analysis has been fundamental in corroborating factors associated with viral reactivation, such as phorbol ester, calcium ionophore and biological stimulation through TGF-β, anti-immunoglobulin, hypoxia, reactive oxygen species (ROS) and temperature changes. Likewise, various epigenetic factors also influence the EBV lytic activation process, mainly histone methylation in different promoters of early virus genes (78). It has been described that plasmodium falciparum can trigger the reactivation process, with the participation of the interdomain region, rich in 1α cysteine, in its membrane protein-1, since it is responsible for activating memory B lymphocytes infected with EBV (79).
Other co-infections favor the EBV reactivation process, such as HPV and cytomegalovirus. Since the recent pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), it has also been described that it can influence the reactivation of EBV, not only in the acute phase of the disease, but also in already recovered patients (80).
On the other hand, ROS are considered a cellular stress factor which cause EBV reactivation. Moreover, LMP1 has been reported to increase ROS production (81). Another mechanism of EBV reactivation is radiation, and it has been reported that a dose of 2 Gy of γ-radiation induces BZLF1 transcription (82).
In addition to the cellular stress factors already mentioned, other stressors have been reported, such as psychosocial ones (mood changes, exposure to excessive noise, family dysfunction) that influence the proper functioning of the immune system via various mechanisms, and may thus also be related to the EBV reactivation process (83) (Fig. 6).
9. Immune response and breast cancer
The transformation of the cells to a phenotype and genotype different from that of their normal lineage, acquire antigenicity that is detected by the immune system, generating a specific antigenic response through the proliferation of lymphocytes. This immunity is acquired when antigen-presenting cells (dendritic cells, CD4+ helper T-cells, and CD8+ cytotoxic T-cells) detect the transformed cells, triggering the release of cytokines, chemokines and lymphokines that contribute to the regulation of immune and inflammatory reactions. There is increasing evidence to indicate that the tumor microenvironment (TME) and inflammation are crucial in the initiation, progression, and response to cancer treatment.
TME elements have cellular components (e.g., fibroblasts, endothelial cells, immune cells, adipocytes) and non-cellular components (e.g., extracellular matrix, growth factors, cytokines, pH), that are critical for understanding tumor biology (84). It is important to analyze the effect of all these immune molecules involved in the oncological process and their value as therapeutic targets in BC, as this may provide valuable information for the development of personalized therapeutic strategies.
TME, inflammation and cytokines
Tumors are known to evade the immune response through the expression of factors that first attract immune cells and subsequently disrupt their recognizing ability, in order to contribute to tumor survival. It has been documented that the human breast tumor microenvironment generates T helper type 2 (Th2) inflammation, a chronic inflammation that promotes the survival and metastasis of cancer cells, through regulation of Toll-like receptor signals, ligands and cytokines (85,86). Tumor-infiltrating CD4+ T-cells that produce type 2 cytokines, such as IL-4, IL-5, IL-9, and IL-13, have an impact on the progression to metastatic BC (87).
Tumor-derived IL-1α is able to induce the expression of a critical factor for tumor survival and metastasis, the cytokine thymic stromal lymphopoietin (TSLP), derived from infiltrating myeloid cells, which results in higher expression of Bcl-2 (anti-apoptotic molecule) (88). The other effect of TSLP is to induce OX40L expression in dendritic cells, promoting the development of IL-13-producing inflammatory Th2 cells and tumor necrosis factors in vitro (86). On the other hand, high levels of IL1-β in tumor-associated inflammation in BC, are produced in CD11c+ cells that infiltrate the tumor, increasing the risk of resistance to treatment and metastasis (89). Immune cells, including macrophages, T-lymphocytes and natural killer cells, produce IL-10, an immunoregulatory cytokine with immunosuppressive and antiangiogenic functions, which promotes tumor cell proliferation and metastasis through the synthesis of TNF, IL-1, IL-12 and chemokines. IL-10 also induces the downregulation of the surface costimulatory molecules, CD80 and CD86, in tumors (82). However, IL-10 inhibits tumorigenesis through the downregulation of VEGF, IL-1b, TNF-α, IL-6 and MMP-9, and additionally inhibits the translocation of NF-κB. Therefore, IL-10 has multifunctional properties, with a dual function, namely as a tumor promoter and inhibitor (90). The activation of this pathway through tripartite motif protein (TRIM47) overexpression in estrogen receptor-positive BC, suggests that protein kinase C epsilon (PKC-ε) and protein kinase D3 (PKD3), known as NF-κB activating protein kinase, are directly associated with TRIM47. The ternary complex with PKC-ε and PKD3, as well as their associated kinases, facilitates the proliferation of BC, conferring resistance to endocrine therapy (tamoxifen); therefore, these molecules can be considered as a future therapeutic target in BC refractory to endocrine therapy (91).
GP130 co-receptor is utilized by IL-6, for the activation of signaling pathways, such as NOTCH, estrogens, P13K and HER2 neu, to favor cell proliferation and metastasis in BC. It has been documented that estrogen-negative BC tumors have higher expression levels of IL-6 compared to estrogen-positive ones (92).
It has been reported that immune dysfunction goes beyond the tumor and can affect distant lymphoid organs. Reports show that cytokine signaling (IFNγ) induces monocyte differentiation into the M1 immunostimulatory phenotype and reverses the immunosuppressive functions of tumor-associated macrophages. IFNγ signals, through the IFNγR1/IFNγR2 complex, activate immune cells through STAT1 phosphorylation, which causes IFN dimerization and translocation to the nucleus to activate IFN-stimulated gene transcription. These findings suggest that cancer-induced systemic immunological changes cause impaired cytokine signaling in peripheral blood monocytes and T-cells at their site of origin, and as a consequence, a possible relapse in BC (93).
The majority of drugs used in chemotherapy for BC are metabolized by cytochrome P450 (CYP), although some epigenetic factors (age, sex, obesity and exercise, among others) have been shown to modify the effectiveness of this enzymatic group, modifying response rates to chemotherapy. It has been verified that, in obese patients, higher levels of IL-6, TNF-α and IL-1β cause modifications in the TME, favoring metastasis and a decreased response to conventional therapies (94). Further studies are required to determine whether CYP expression and activity inhibition is caused by high levels of circulating inflammatory cytokines during chemotherapy in BC.
10. Therapeutic targets and personalized medicine
In the search for novel alternative therapies for drug-resistant tumors, BC stem cells (BCSC) have emerged as targets. Cytokines in the TME can regulate BCSC self-renewal and survival via various mechanisms; therefore, the blocking of certain cytokines (IL-6 and IL-8) may be a novel therapeutic strategy (95). On the other hand, tumor-associated macrophages in BC that present a high expression of insulin-associated growth factors type I and II are the main source for primary and metastatic tumor growth. All these molecules represent potential therapeutic targets, since blocking them, plus conventional chemotherapy with taxanes can lead to improved results (96). Cases of BC with a poor response to treatment and metastasis have been related to IL-1β overexpression (89). In this regard, studies blocking this cytokine (pilot clinical trial NCT01802970) have been carried out, although in phase I, it has shown encouraging results (89). As already mentioned, the molecular process mediated by IL-1 include the inflammatory reactions of organs and cells, immune responses and homeostatic regulation, which confirms its participation in oncogenesis, invasion, and metastasis through a perpetuating condition with chronic proinflammatory state. Due to the immense therapeutic potential attributed to it, IL-1 inhibition has been used in BC, where gevokizumab, bermekimab and nidanilimab antibodies stand out with promising results in metastatic BC (97).
IL-1-mediated inflammatory signaling is involved in immunosuppression and immune escape through the production and maintenance of an inflammatory microenvironment, which leads to the progression of BC. Thus, blocking abnormal IL-1 signaling caused by a tumor can be used as immunotherapy or adjuvant immunotherapy to reduce inflammation/immunosuppression and enhance antitumor immunity.
The expression by cancer cells of programmed death ligand 1 (PD-L1), also known as CD274, plays a crucial role in the suppression of the immune system response; thus, it has been considered a therapeutic target in BC when overexpression is identified. However, PD-L1 is still under investigation due to the variability in expression and results as shown in different studies (98).
Intracellular adhesion molecule 1 (ICAM1) is overexpressed in triple-negative and HER2 neu BC as part of the response in the TME; however, its expression also increases by TNFα, IL-1β and IFNγ stimulation in normal mammary cells. ICAM1 is a critical pathway involved in cell proliferation, adhesion and dissemination in high-grade breast tumors through its interaction with tertiary lymphoid structures that are responsible for tumor neovascularization in BC and multiple solid tumors. According to this, although ICAM1 biological importance remains controversial, it is considered a promising future therapeutic target in triple-negative and HER2 neu BC (99).
Finally, it is important to emphasize the necessity of studies including gene and epigenetic regulatory mechanisms, as well as those using multi-omics technology approaches to know the immuno-oncological interactions and the mechanisms at the cellular, molecular and nuclear level exerted by the cytokines involved, to promote the development of more effective therapies for BC (100).
The scenario of EBV-related BC is very broad. Known risk factors for this neoplasm are involved, in addition to those that add up once the malignant process has started, affecting the development of the disease.
11. Summary of Epstein-Barr virus-associated oncogenesis
Although several decades have passed since EBV and its association to various diseases were first described, information about the virus continues to increase, demonstrating multiple functions of the various viral components and how they influence various relationships with associated diseases, which undoubtedly improves the understanding in this regard.
EBV presents various adaptation mechanisms that make it a perfect tiny machinery to evade the host's defense mechanisms and thus perpetuate its effects. A number of these mechanisms are not entirely clear and some are even currently unknown; these include not only EBV but most viruses; proof of this is the devastating effect or damage they can cause in human beings, as evidenced by the SARS-CoV-2 pandemic.
Despite the fact that EBV has one of the highest prevalence in worldwide, only a very low percentage of individuals will present clinical manifestations, which reveals that a competent immune system in the host is crucial to limit EBV pathogenic or oncogenic potential. The foregoing is related to the latency pattern that the virus acquires or presents in each of the cases, since the greater the participation or expression of the majority of the viral genes, the greater the effect or repercussions on the host. However, these latency phases, being different for each disease or associated neoplasia, may be the indicator of the requirements that the virus needs or establishes. Leaving as a common denominator, the role of viral components for immune evasion, it may manifest itself or wait for the ideal conditions or necessary stimuli.
EBV is considered an oncovirus in BC, due to the epigenetic and genetic functions of its multiple miRNAs and lncRNAs, exerting its effects in a timely manner with various mechanisms to evade the immune system, infecting epithelia and, together with other factors, promoting malignant processes.
In BC, as an epithelial neoplasm, the mechanism of infection is more complex than that of lymphoproliferative diseases. It has been given a specific latency pattern; nonetheless, since there are no conclusive references in this regard, due to the fact that the majority of studies are not specific, it leaves open the possibility that the virus may present variants in terms of latency states and that this may influence disease behavior. Even if this multifactorial disease is influenced by genetic, epigenetic, hormonal and environmental aspects, among others, it may present multiple latency patterns or at least one that is not conventional to what has been described.
For all of the above, some reports even consider EBV infection to be an added risk factor for developing BC, with shorter disease-free periods and being associated with high-grade tumors and greater aggressiveness, such as triple-negative and HER 2 neu BC. In this regard, the studies by Farahmand et al (101) and Jin et al (102) stand out, with an odds ratio of 4.7 (both studies), which appears to be conclusive for this disease.
However, the behavior of the virus or its influence is not similar in all populations, highlighting that for very particular geographical areas in Asia and Africa, the population appears to be more susceptible to this infection. Perhaps the type of EBV that prevails in these areas should be considered as a first possibility, as well as the multiple co-infections that prevail in these developing countries. A study conducted in Mexican women did not reveal an association between BC and the presence of EBV (103). However, one of the limitations was that it only involved the detection of the virus without considering a latency analysis and evidence of the expression patterns of viral proteins with tumor activity. On the other hand, the sample is small, so it was not conclusive for Mexican population (103).
In addition, the effects of another virus, including SARS-CoV-2, will have to be added, which will become evident as time goes by.
Due to the high prevalence of EBV in the global population, variants or mutations in its genome undoubtedly represent one of the possible causes for which clinical or oncogenic manifestations only occur in some hosts. DNA viruses such as EBV generally have fairly stable genomic sequences, but there are some variations between different parts of the world that may explain the different incidences of EBV-associated diseases, including cancer. There is evidence of virus reassortment in EBV, although it is difficult to define EBV evolutionary lineages due to the uneven distribution of polymorphisms and recombination between strains (104,105).
Variation at defined nucleotide positions in the EBV genome is known to be characteristic of the geographic region of origin of EBV strains, although there is uncertainty about the factors that are responsible for these differences. It is useful to note that the direct sequencing of EBV DNA from healthy, normal and persistently infected individuals has revealed that they usually contain a predominant single EBV sequence, although there may also be low levels of alternative sequences, indicating strain mixtures in some individuals. It is not known whether these mixtures result from initial infection with a mixture of strains or are due to superinfection during normal social contact (106).
The foregoing denotes the importance and complexity generated by EBV mutations or polymorphisms, and how this could have direct repercussions on the manifestations or presentations of the various diseases or neoplasms associated with this virus.
Undoubtedly, the prevalence of EBV in numerous geographical settings is related to socioeconomic status, since in certain regions of the planet, it is inherent to high rates of malnutrition. This goes hand in hand with a weak immune system, limited access to or no health systems, together representing ideal conditions for the invasion with other viruses, including EBV and other diseases.
The scenario for EBV-related BC is very broad, in which known risk factors for this neoplasm are involved, in addition to those that are added once the malignant process has begun, such as the effects of infection or co-infections that favor the lytic phase and affect the behavior of the disease (Fig. 7).
12. Conclusions and future perspectives
The present review described the role of miRNAs, lncRNAs and the various EBV genes that involve various epigenetic processes, such as the silencing of tumor suppressor genes, the regulation of apoptosis and the control of oncogenic proteins, establishing a tumor-promoting environment and resistance to conventional treatments. It is demonstrated that the interaction of various additional factors (environmental and host) is required in the evolution and presentation of BC associated with EBV infection, highlighting the predisposition by heredity, race, co-infections and the immunological status of the host.
It is necessary to consider other factors, such as individual genetic features, the possible association between proteins and molecules expressed from tumor cells, elements of the tumor environment, plus epigenetic alterations and/or polymorphisms of tumor suppressor genes, as well as immunological mechanisms affecting the ability of recognition of the immune response and population/ethnicity characteristics.
Currently, research is leading to immunopharmacology and pharmacogenetic development, since the knowledge generated using technology with multi-omics approaches has the purpose of creating new therapeutic schemes based on personalized medicine. In this sense, immunotherapy in BC appears promising, since several therapeutic targets have been identified. Although these targets are not yet fully validated, the encouraging results shown are worthy of further in-depth study.
There is still a long way to go, since these personalized therapies will require innovative drug delivery systems, as well as the generation of knowledge leading to more and precise understanding of mechanisms involved in oncogenesis, metastasis and non-response to BC therapies.
The process of lytic reactivation, with inherent gene expression, may or may not be necessary to modify the course of EBV-associated disease. Therefore, it would be crucial to determine, as far as possible, the latency pattern for BC. Finally, although the role played by the association between EBV and BC has been studied, it remains controversial and the findings reported on the viral components that participate in the evolution of the disease should not be ignored, and the investigation should continue.
Availability of data and materials
Not applicable.
Authors' contributions
JDGA, IPL were involved in the design of the study and in content development. JAG, CPR, AMEG, PGAT, JXC, SAO, ACC performed the review of articles to be included in each chapter and prepared the corresponding figures. Data authentication is not applicable. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
The present review was carried out in collaboration with José Damián Gómez Archila, doctoral fellow in the Postgraduate Program of Doctorate in Research in Medicine of the National Polytechnic Institute and CONACyT program/1032676. The authors appreciate the collaboration of Luis Javier Cervantes Palma, Bachelor of Tourism and Sustainable Development, freelance designer.
Funding
The present study was supported by the direction of research at the Hospital Infantil de México Federico Gómez HIM/2021/072 and Escuela Superior de Medicina IPN Proyecto SIP No. 20220938.
References
|
Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, et al: A review of human carcinogens-Part B: biological agents. Lancet Oncol. 10:321–322. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Muñoz N, Castellsagué X, Berrington de González A and Gissmann L: Chapter 1: HPV in the etiology of human cancer. Vaccine. 24(Suppl 3): S1–S10. 2006. View Article : Google Scholar | |
|
Bialecki ES and Di Bisceglie AM: Clinical presentation and natural course of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 17:485–489. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Hermine O, Lefrère F, Bronowicki JP, Mariette X, Jondeau K, Eclache-Saudreau V, Delmas B, Valensi F, Cacoub P, Brechot C, et al: Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med. 347:89–94. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Thompson MP and Kurzrock R: Epstein-Barr virus and cancer. Clin Cancer Res. 10:803–821. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Mesri EA, Feitelson MA and Munger K: Human viral oncogenesis: A cancer hallmarks analysis. Cell Host Microbe. 15:266–282. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Medina-Ortega AP, López-Valencia D, Mosquera-Monje SL, Mora-Obando DL and Dueñas-Cuéllar RA: Relationship between Epstein-Barr virus and cancer development. Iatreia. 30:131–145. 2017. View Article : Google Scholar | |
|
Pietropaolo V, Prezioso C and Moens U: Role of virus-induced host cell epigenetic changes in cancer. Int J Mol Sci. 22:83462021. View Article : Google Scholar : PubMed/NCBI | |
|
Hulse M, Caruso LB, Madzo J, Tan Y, Johnson S and Tempera I: Poly(ADP-ribose) polymerase 1 is necessary for coactivating hypoxia-inducible factor-1-dependent gene expression by Epstein-Barr virus latent membrane protein 1. PLoS Pathog. 4:e10073942018. View Article : Google Scholar | |
|
Cao Y, Xie L, Shi F, Tang M, Li Y, Hu J, Zhao L, Zhao L, Yu X, Luo X, et al: Targeting the signaling in Epstein-Barr virus-associated diseases: Mechanism, regulation, and clinical study. Signal Transduct Target Ther. 6:152021. View Article : Google Scholar : PubMed/NCBI | |
|
He B, Li W, Wu Y, Wei F, Gong Z, Bo H, Wang Y, Li X, Xiang B, Guo C, et al: Epstein-Barr virus-encoded miR-BART6-3p inhibits cancer cell metastasis and invasion by targeting long non-coding RNA LOC553103. Cell Death Dis. 7:e23532016. View Article : Google Scholar : PubMed/NCBI | |
|
Hechter O, Sausen DG, Gallo ES, Dahari H and Borenstein R: Epstein-Barr virus (EBV) epithelial associated malignancies: Exploring pathologies and current treatments. Int J Mol Sci. 23:143892022. View Article : Google Scholar | |
|
Feng Y, Spezia M, Huang S, Yuan C, Zeng Z, Zhang L, Ji X, Liu W, Huang B, Luo W, et al: Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 5:77–106. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Wang M, Yu F, Wu W, Wang Y, Ding H and Qian L: Epstein-Barr virus-encoded microRNAs as regulators in host immune responses. Int J Biol Sci. 14:565–576. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
El-Sharkawy A, Al Zaidan L and Malki A: Epstein-Barr virus-associated malignancies: Roles of viral oncoproteins in carcinogenesis. Front Oncol. 8:2652018. View Article : Google Scholar : PubMed/NCBI | |
|
Shannon-Lowe C, Adland E, Bell A, Delecluse H, Rickinson A and Rowe M: Features distinguishing Epstein-Barr virus infections of epithelial cells and B cells: viral genome expression, genome maintenance, and genome amplification. J Virol. 83:7749–7760. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Sinclair AJ, Moalwi MH and Amoaten T: Is EBV associated with breast cancer in specific geographic locations? Cancers (Basel). 13:8192021. View Article : Google Scholar : PubMed/NCBI | |
|
Shannon-Lowe C and Rowe M: Epstein Barr virus entry; kissing and conjugation. Curr Opin Virol. 4:78–84. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Shannon-Lowe C and Rowe M: Epstein-Barr virus infection of polarized epithelial cells via the basolateral surface by memory B cell-mediated transfer infection. PLoS Pathog. 7:e10013382011. View Article : Google Scholar : PubMed/NCBI | |
|
Imai S, Nishikawa J and Takada K: Cell-to-cell contact as an efficient mode of Epstein-Barr virus infection of diverse human epithelial cells. J Virol. 72:4371–4378. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Tugizov SM, Berline JW and Palefsky JM: Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat Med. 9:307–314. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Chen J, Sathiyamoorthy K, Zhang X, Schaller S, Perez White BE, Jardetzky TS and Longnecker R: Ephrin receptor A2 is a functional entry receptor for Epstein-Barr virus. Nat Microbiol. 3:172–180. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang H, Li Y, Wang HB, Zhang A, Chen ML, Fang ZX, Dong XD, Li SB, Du Y, Xiong D, et al: Ephrin receptor A2 is an epithelial cell receptor for Epstein-Barr virus entry. Nat Microbiol. 3:164–171. 2018. | |
|
Ayee R, Ofori MEO, Wright E and Quaye O: Epstein Barr Virus associated lymphomas and epithelia cancers in humans. J Cancer. 11:1737–1750. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Al Moustafa AE, Al-Antary N, Aboulkassim T, Akil N, Batist G and Yasmeen A: Co-prevalence of Epstein-Barr virus and high-risk human papillomaviruses in Syrian women with breast cancer. Hum Vaccin Immunother. 12:1936–1939. 2016.PubMed/NCBI | |
|
Lin JH, Tsai CH, Chu JS, Chen JY, Takada K and Shew JY: Dysregulation of HER2/HER3 signaling axis in Epstein-Barr virus-infected breast carcinoma cells. J Virol. 81:5705–5713. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Pal AD, Basak NP, Banerjee AS and Banerjee S: Epstein-Barr virus latent membrane protein-2A alters mitochondrial dynamics promoting cellular migration mediated by Notch signaling pathway. Carcinogenesis. 35:1592–1601. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Arias-Calvachi C, Blanco R, Calaf GM and Aguayo F: Epstein-Barr virus association with breast cancer: Evidence and perspectives. Biology (Basel). 11:7992022.PubMed/NCBI | |
|
Nasser F, Moussa N, Helmy MW and Haroun M: Dual targeting of Notch and Wnt/β-catenin pathways: Potential approach in triple-negative breast cancer treatment. Naunyn Schmiedebergs Arch Pharmacol. 394:481–490. 2021. View Article : Google Scholar | |
|
Gómez-Archila JD, Espinosa-García AM, Palacios-Reyes C, Trujillo-Cabrera Y, Mejía ALS, González AVA, Rangel-López E, Alonso-Themann PG, Solís NDS, Hernández-Zavala A, et al: NOTCH expression variability and relapse of breast cancer in high-risk groups. Am J Med Sci. 364:583–594. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Shahi V, Agarwal P, Qayoom S, Kumar V, Tewari S, Raghuvanshi S, Singh US and Goel MM: Detection of Epstein Barr nuclear antigen-1 (EBNA-1), early antigen 1F, 2R (EA-1F, EA-2R) along with Epstein-Barr virus latent membrane protein 1 (LMP1) in breast cancer of Northern India: An interim analysis. Asian Pac J Cancer Prev. 23:3717–3723. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Z, Li Z, Wu Q, Li C, Li J, Zhang Y, Wang C and Sun S and Sun S: DNER promotes epithelial-mesenchymal transition and prevents chemosensitivity through the Wnt/β-catenin pathway in breast cancer. Cell Death Dis. 11:6422020. View Article : Google Scholar | |
|
Zimber-Strobl U and Strobl LJ: EBNA2 and Notch signalling in Epstein-Barr virus mediated immortalization of B lymphocytes. Semin Cancer Biol. 11:423–434. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Anderson L and Longnecker R: An auto-regulatory loop for EBV LMP2A involves activation of Notch. Virology. 371:257–266. 2008. View Article : Google Scholar | |
|
Cyprian FS, Al-Farsi HF, Vranic S, Akhtar S and Al Moustafa AE: Epstein-Barr virus and human papillomaviruses interactions and their roles in the initiation of epithelial-mesenchymal transition and cancer progression. Front Oncol. 8:1112018. View Article : Google Scholar : PubMed/NCBI | |
|
Zanella L, Riquelm I, Buchegger K, Abanto M, Ili C and Brebi P: A reliable Epstein-Barr virus classification based on phylogenomic and population analyses. Sci Rep. 9:98292019. View Article : Google Scholar : PubMed/NCBI | |
|
Zimber U, Adldinger HK, Lenoir GM, Vuillaume M, Knebel-Doeberitz MV, Laux G, Desgranges C, Wittmann P, Freese UK, Schneider U, et al: Geographical prevalence of two types of Epstein-Barr virus. Virology. 154:56–66. 1986. View Article : Google Scholar : PubMed/NCBI | |
|
Smith NA, Baresel PC, Jackson CL, Ogolla S, Toko EN, Heit S, Piriou E, Sumba OP, Middeldorp JM, Colborn KL and Rochford R: Differences in the Epstein-Barr virus gp350 IgA antibody response are associated with increased risk for coinfection with a second strain of Epstein-Barr virus. J Infect Dis. 219:955–963. 2019. View Article : Google Scholar : | |
|
Lung ML, Li SB and Chang RS: Study of Epstein-Barr virus (EBV) transmission by EBV genotyping. J Infect Dis. 164:213–214. 1991. View Article : Google Scholar : PubMed/NCBI | |
|
Golrokh Mofrad M, Kazeminezhad B and Faghihloo E: Prevalence of Epstein-Barr virus (EBV) in Iranian breast carcinoma patients. Asian Pac J Cancer Prev. 21:133–137. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Gupta I, Jabeen A, Al-Sarraf R, Farghaly H, Vranic S, Sultan AA, Al Moustafa AE and Al-Thawadi H: The co-presence of high-risk human papillomaviruses and Epstein-Barr virus is linked with tumor grade and stage in Qatari women with breast cancer. Hum Vaccin Immunother. 17:982–989. 2021. View Article : Google Scholar : | |
|
Aboulkassim T, Yasmeen A, Akil N, Batist G and Al Moustafa AE: Incidence of Epstein-Barr virus in Syrian women with breast cancer: A tissue microarray study. Hum Vaccin Immunother. 11:951–955. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Fessahaye G, Elhassan AM, Elamin EM, Adam AAM, Ghebremedhin A and Ibrahim ME: Association of Epstein-Barr virus and breast cancer in Eritrea. Infect Agents Cancer. 12:622017. View Article : Google Scholar | |
|
Nwagu GC, Bhattarai S, Swahn M, Ahmed S and Aneja R: Prevalence and mortality of triple-negative breast cancer in West Africa: Biologic and sociocultural factors. JCO Glob Oncol. 7:1129–1140. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Acheampong T, Kehm RD, Terry MB, Argov EL and Tehranifar P: Incidence trends of breast cancer molecular subtypes by age and race/ethnicity in the US from 2010 to 2016. JAMA Netw Open. 3:e20132262020. View Article : Google Scholar : PubMed/NCBI | |
|
Siddharth S and Sharma D: Racial disparity and triple-negative breast cancer in African-American women: A multifaceted affair between obesity, biology, and socioeconomic determinants. Cancers (Basel). 10:5142018. View Article : Google Scholar : PubMed/NCBI | |
|
Ibrahim SA, Hassan H, Vilardo L, Kumar SK, Kumar AV, Kelsch R, Schneider C, Kiesel L, Eich HT, Zucchi I, et al: Syndecan-1 (CD138) modulates triple-negative breast cancer stem cell properties via regulation of LRP-6 and IL-6-mediated STAT3 signaling. PLoS One. 8:e857372013. View Article : Google Scholar | |
|
Rahim A, Afzal M and Naveed AK: Genetic polymorphism of miRNA-196a and its target gene annexin-A1 expression based on ethnicity in Pakistani female breast cancer patients. Pak J Med Sci. 35:1598–1604. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Treece AL, Duncan DL, Tang W, Elmore S, Morgan DR and Gulley ML, Speck O, Meyers MO and Gulley ML: Gastric adenocarcinoma microRNA profiles in fixed tissue and in plasma reveal cancer-associated and Epstein-Barr virus-related expression patterns. Lab Invest. 96:661–671. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Ramanto KN, Widianto KJ, Wibowo SSH and Agustriawan D: The regulation of microRNA in each of cancer stage from two different ethnicities as potential biomarker for breast cancer. Comput Biol Chem. 93:1074972021. View Article : Google Scholar : PubMed/NCBI | |
|
GLOBOCAN 2020: International Agency for Research of Cancer 2022. Available in: http://gco.iarc.fr/. Accessed May 5, 2022 | |
|
Mexican consensus on diagnosis and breast cancer treatment. GAMO. 7(Supl 2)2021. View Article : Google Scholar | |
|
Smatti MK, Al-Sadeq DW, Ali NH, Pintus G, Abou-Saleh H and Nasrallah GK: Epstein-Barr virus epidemiology, serology, and genetic variability of LMP-1 oncogene among healthy population: An update. Front Oncol. 8:2112018. View Article : Google Scholar : PubMed/NCBI | |
|
Fierti AO, Yakass MB, Okertchiri EA, Adadey SM and Quay O: The role of Epstein-Barr virus in modulating key tumor suppressor genes in associated malignancies: Epigenetics, transcriptional, and post-translational modifications. Biomolecules. 12:1272022. View Article : Google Scholar : PubMed/NCBI | |
|
Mátyási B, Farkas Z, Kopper L, Sebestyén A, Boissan M, Mehta A and Takács-Vellai K: The function of NM23-H1/NME1 and its homologs in major processes linked to metastasis. Pathol Oncol Res. 26:49–61. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Cheerathodi MR and Meckes DG Jr: The Epstein-Barr virus LMP1 interactome: Biological implications and therapeutic targets. Future Virol. 13:863–887. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Longnecker R and Kieff E: A second Epstein-Barr virus membrane protein (LMP2) is expressed in latent infection and colocalizes with LMP1. J Virol. 64:2319–2326. 1990. View Article : Google Scholar : PubMed/NCBI | |
|
Wasil LR and Shair KHY: Modified anoikis assay that functionally segregates Epstein-Barr virus LMP1 strains into two groups. J Virol. 92:e00557–18. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Xie L, Shi F, Li Y, Li W, Yu X, Zhao L, Zhou M, Hu J, Luo X, Tang M, et al: Drp1-dependent remodeling of mitochondrial morphology triggered by EBV-LMP1 increases cisplatin resistance. Signal Transduct Target Ther. 5:562020. View Article : Google Scholar : PubMed/NCBI | |
|
Pratt ZL, Kuzembayeva M, Sengupta S and Sugden B: The microRNAs of Epstein-Barr virus are expressed at dramatically differing levels among cell lines. Virology. 386:387–397. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Wang M, Gu B, Chen X, Wang Y, Li P and Wang K: The function and therapeutic potential of Epstein-Barr virus-encoded MicroRNAs in cancer. Mol Ther Nucleic Acids. 17:657–668. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Y, Hu Z, Zhang Y and Wang C: Long non-coding RNAs in Epstein-Barr virus-related cancer. Cancer Cell Int. 21:2782021. View Article : Google Scholar : PubMed/NCBI | |
|
Kang MS and Kieff E: Epstein-Barr virus latent genes. Exp Mol Med. 47:e1312015. View Article : Google Scholar : PubMed/NCBI | |
|
Houmani JL, Davis CI and Ruf IK: Growth-promoting properties of Epstein-Barr virus EBER-1 RNA correlate with ribosomal protein L22 binding. J Virol. 83:9844–9853. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Samanta M, Iwakiri D, Kanda T, Imaizumi T and Takada K: EB virus-encoded RNAs are recognized by RIG-I and activate signaling to induce type I IFN. EMBO J. 25:4207–4214. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Yang GD, Huang TJ, Peng LX, Yang CF, Liu RY, Huang HB, Chu QQ, Yang HJ, Huang JL, Zhu ZY, et al: Epstein-Barr virus_encoded LMP1 upregulates microRNA-21 to promote the resistance of nasopharyngeal carcinoma cells to cisplatin-induced apoptosis by suppressing PDCD4 and Fas-L. PLoS One. 8:e783552013. View Article : Google Scholar : PubMed/NCBI | |
|
Sales ACV, Gomes da Silva IIF, Leite MCB, Coutinho LL, Reis RBAC, Castoldi A, Bg Martins D, Lima-Filho JL and Souto FO: Mirna21 expression in the breast cancer tumor tissue is independent of neoadjuvant chemotherapy. Breast Cancer (Dove Med Press). 12:141–151. 2020.PubMed/NCBI | |
|
Zhu K, Wu Y, He P, Fan Y, Zhong X, Zheng H and Luo T: PI3K/AKT/mTOR-targeted therapy for breast cancer. Cells. 11:25082022. View Article : Google Scholar : PubMed/NCBI | |
|
Tankova T, Senkus E, Beloyartseva M, Borštnar S, Catrinoiu D, Frolova M, Hegmane A, Janež A, Krnić M, Lengyel Z, et al: Management strategies for hyperglycemia associated with the α-selective PI3K inhibitor alpelisib for the treatment of breast cancer. Cancers (Basel). 14:15982022. View Article : Google Scholar | |
|
Mostafaei S, Kazemnejad A, Norooznezhad AH, Mahaki B and Moghoofei M: Simultaneous effects of viral factors of human papilloma virus and Epstein-Barr virus on progression of breast and thyroid cancers: Application of structural equation modeling. Asian Pac J Cancer Prev. 21:1431–1439. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Tsai JH, Tsai CH, Cheng MH, Lin SJ, Xu FL and Yang CC: Association of viral factors with non-familial breast cancer in Taiwan by comparison with non-cancerous, fibroadenoma, and thyroid tumor tissues. J Med Virol. 75:276–281. 2005. View Article : Google Scholar | |
|
Tsai JH, Hsu CS, Tsai CH, Su JM, Liu YT, Cheng MH, Wei JC, Chen FL and Yang CC: Relationship between viral factors, axillary lymph node status and survival in breast cancer. J Cancer Res Clin Oncol. 133:13–21. 2007. View Article : Google Scholar | |
|
Klein G: Tumor associations of EBV-historical perspectives. Curr Top Microbiol Immunol. 390:17–22. 2015. | |
|
Yates J, Warren N, Reisman D and Sugden B: A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci USa. 81:3806–3810. 1984. View Article : Google Scholar : PubMed/NCBI | |
|
Naushad W, Surriya O and Sadia H: Prevalence of EBV, HPV and MMTV in Pakistani breast cancer patients: A possible etiological role of viruses in breast cancer. Infect Genet Evol. 54:230–237. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Corbex M, Bouzbid S, Traverse-Glehen A, Aouras H, McKay-Chopin S, Carreira C, Lankar A, Tommasino M and Gheit T: Prevalence of papillomaviruses, polyomaviruses, and herpesviruses in triple-negative and inflammatory breast tumors from Algeria compared with other types of breast cancer tumors. PLoS One. 9:e1145592014. View Article : Google Scholar : PubMed/NCBI | |
|
McKenzie J and El-Guindy A: Epstein-Barr virus lytic cycle reactivation. Curr Top Microbiol Immunol. 391:237–261. 2015.PubMed/NCBI | |
|
Murata T, Sugimoto A, Inagaki T, Yanagi Y, Watanabe T, Sato Y and Kimura H: Molecular basis of Epstein-Barr virus latency establishment and lytic reactivation. Viruses. 13:23442021. View Article : Google Scholar : PubMed/NCBI | |
|
Chéne A, Donati D, Guerreiro-Cacais AO, Levitsky V, Chen Q, Falk KI, Orem J, Kironde F, Wahlgren M and Bejarano MT: A molecular link between malaria and Epstein-Barr virus reactivation. PLoS Pathog. 3:e802007. View Article : Google Scholar : PubMed/NCBI | |
|
Gold JE, Okyay RA, Licht WE and Hurley DJ: Investigation of long COVID prevalence and its relationship to Epstein-Barr virus reactivation. Pathogens. 10:7632021. View Article : Google Scholar : PubMed/NCBI | |
|
Hu J, Li Y, Li H, Shi F, Xie L, Zhao L, Tang M, Luo X, Jia W, Fan J, et al: Targeting Epstein-Barr virus oncoprotein LMP1-mediated high oxidative stress suppresses EBV lytic reactivation and sensitizes tumors to radiation therapy. Theranostics. 10:11921–11937. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Mehta SK, Bloom DC, Plante I, Stowe R, Feiveson AH, Renner A, Dhummakupt A, Markan D, Zhang Y, Wu H, et al: Reactivation of latent Epstein-Barr virus: A comparison after exposure to gamma, proton, carbon, and iron radiation. Int J Mol Sci. 19:29612018. View Article : Google Scholar : PubMed/NCBI | |
|
Sausen DG, Bhutta MS, Gallo ES, Dahari H and Borenstein R: Stress-induced Epstein-Barr virus reactivation. Biomolecules. 11:13802021. View Article : Google Scholar : PubMed/NCBI | |
|
Anderson NM and Simon MC: The tumor microenvironment. Curr Biol. 30:R921–R925. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Kluwe J, Mencin A and Schwabe RF: Toll-like receptors, wound healing, and carcinogenesis. J Mol Med (Berl). 87:125–138. 2009. View Article : Google Scholar | |
|
Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C, Tindle S, Marches F, Gallegos M, Burton EC, Savino D, Hori T, et al: Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med. 208:479–490. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N and Coussens LM: CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 16:91–102. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Kuan EL and Ziegler SF: A tumor-myeloid cell axis, mediated via the cytokines IL-1α and TSLP, promotes the progression of breast cancer. Nat Immunol. 19:366–374. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Wu TC, Xu K, Martinek J, Young RR, Banchereau R, George J, Turner J, Kim KI, Zurawski S, Wang X, et al: IL1 receptor antagonist controls transcriptional signature of inflammation in patients with metastatic breast cancer. Cancer Res. 78:5243–5258. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Sheikhpour E, Noorbakhsh P, Foroughi E, Farahnak S, Nasiri R and Neamatzadeh H: A survey on the role of interleukin-10 in breast cancer: A narrative. Rep Biochem Mol Biol. 7:30–37. 2018.PubMed/NCBI | |
|
Azuma K, Ikeda K, Suzuki T, Aogi K, Horie-Inoue K and Inoue S: TRIM47 activates NF-κB signaling via PKC-ε/PKD3 stabilization and contributes to endocrine therapy resistance in breast cancer. Proc Natl Acad Sci USA. 118:e21007841182021. View Article : Google Scholar | |
|
Omokehinde T and Johnson RW: GP130 cytokines in breast cancer and bone. Cancers (Basel). 12:3262020. View Article : Google Scholar : PubMed/NCBI | |
|
Wang L, Simons DL, Lu X, Tu TY, Avalos C, Chang AY, Dirbas FM, Yim JH, Waisman J and Lee PP: Breast cancer induces systemic immune changes on cytokine signaling in peripheral blood monocytes and lymphocytes. EBioMedicine. 52:1026312020. View Article : Google Scholar : PubMed/NCBI | |
|
Crake RLI, Strother MR, Phillips E, Doogue MP, Zhang M, Frampton CMA, Robinson BA and Currie MJ: Influence of serum inflammatory cytokines on cytochrome P450 drug metabolising activity during breast cancer chemotherapy: A patient feasibility study. Sci Rep. 11:56482021. View Article : Google Scholar : PubMed/NCBI | |
|
Sparano JA, O'Neill A, Graham N, Northfelt DW, Dang CT, Wolff AC, Sledge GW and Miller KD: Inflammatory cytokines and distant recurrence in HER2-negative early breast cancer. NPJ Breast Cancer. 8:162022. View Article : Google Scholar : PubMed/NCBI | |
|
Chen W, Qin Y and Liu S: Cytokines, breast cancer stem cells (BCSCs) and chemoresistance. Clin Transl Med. 7:272018. View Article : Google Scholar : PubMed/NCBI | |
|
Liu F, Li L, Lan M, Zou T, Kong Z, Cai T, Wu XY and Cai Y: Key factor regulating inflammatory microenvironment, metastasis, and resistance in breast cancer: Interleukin-1 signaling. Mediators Inflamm. 2021:77858902021. View Article : Google Scholar : PubMed/NCBI | |
|
Cierna Z, Smolkova B, Cholujova D, Gronesova P, Miklikova S, Cihova M, Plava J and Mego M: Decreased levels of circulating cytokines VEGF, TNF-β and IL-15 indicate PD-L1 overexpression in tumours of primary breast cancer patients. Sci Rep. 11:12942021. View Article : Google Scholar | |
|
Figenschau SL, Knutsen E, Urbarova I, Fenton C, Elston B, Perander M, Mortensen ES and Fenton KA: ICAM1 expression is induced by proinflammatory cytokines and associated with TLS formation in aggressive breast cancer subtypes. Sci Rep. 8:117202018. View Article : Google Scholar : PubMed/NCBI | |
|
Jurisic V: Multiomic analysis of cytokines in immuno-oncology. Expert Rev Proteomics. 17:663–674. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Farahmand M, Monavari SH, Shoja Z, Ghaffari H, Tavakoli M and Tavakoli A: Epstein-Barr virus and risk of breast cancer: A systematic review and meta-analysis. Future Oncol. 15:2873–2885. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Jin Q, Su J, Yan D and Wu S: Epstein-Barr virus infection and increased sporadic breast carcinoma risk: A meta-analysis. Med Princ Pract. 29:195–200. 2020. View Article : Google Scholar : | |
|
Morales-Sánchez A, Molina-Muñoz T, Martínez-López JL, Hernández-Sancén P, Mantilla A, Leal YA, Torres J and Fuentes-Pananá EM: No association between Epstein-Barr virus and mouse mammary tumor virus with breast cancer in Mexican women. Sci Rep. 3:29702013. View Article : Google Scholar : PubMed/NCBI | |
|
Palser AL, Grayson NE, White RE, Corton C, Correia S, Ba Abdullah MM, Watson SJ, Cotton M, Arrand JR, Murray PG, et al: Genome diversity of Epstein-Barr virus from multiple tumor types and normal infection. J Virol. 89:5222–5237. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Ba Abdullah MM, Palermo RD, Palser AL, Grayson NE, Kellam P, Correia S, Szymula A and White RE: Heterogeneity of the Epstein-Barr virus (EBV) major internal repeat reveals evolutionary mechanisms of EBV and a functional defect in the prototype EBV strain B95-8. J Virol. 91:e00920–17. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Farrell PJ and White RE: Do Epstein-Barr virus mutations and natural genome sequence variations contribute to disease? Biomolecules. 12:172021. View Article : Google Scholar |