Role of AMIGO2 in cancer progression: Novel insights (Review)
- Authors:
- Published online on: July 12, 2024 https://doi.org/10.3892/ol.2024.14567
- Article Number: 434
-
Copyright: © Tian et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Metastasis is the hallmark of cancer and is responsible for the majority of cancer-related deaths. However, it still needs to be better understood. Cell adhesion molecules (CAMs), the transmembrane proteins facilitating interactions between cells, and between cells and the extracellular matrix, are involved in controlling cell adhesion of cancer metastasis. There are four types of CAMs: Integrins, cadherins, selectins and the Ig superfamily (1–3). CAMs serve a vital role in diverse biological processes, such as cell adhesion, proliferation, differentiation, apoptosis and signal transduction (4,5). As a member of the CAM family, adhesion molecule with IgG-like domain 2 (AMIGO2) is critical for the cell biological process.
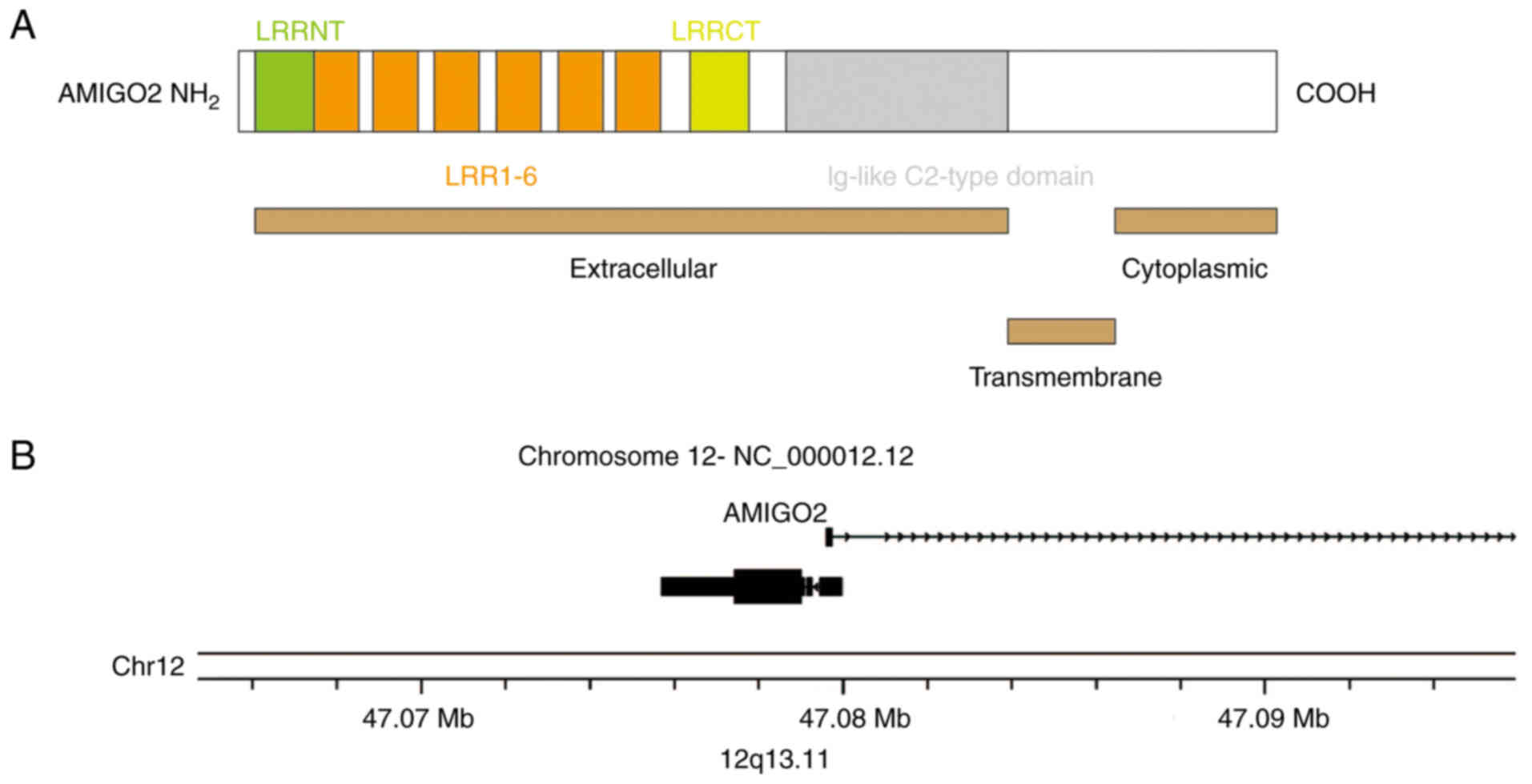
Kuja-Panula et al (6) identified the amphoterin-induced gene and ORF (AMIGO) gene, subsequently cloning two other homologous genes, AMIGO2 and AMIIGO3. The Amigo family proteins all belong to the type-I transmembrane proteins with six leucine-rich repeats and one IgG-like domain in the extracellular region (Fig. 1A). Additionally, they can be mediated by homophilic or heterophilic binding, facilitating cell interactions. Although the Amigo family members have highly similar structures, their expression and biological activities are distinct. AMIGO1 has been implicated in promoting axon growth in the retina (7). Conversely, AMIGO3 and leucine rich repeat and immunoglobin-like domain-containing protein 1 (LINGO-1) have leucine-rich repeat domains, and AMIIGO3 can replace LINGO-1 to form a complex with Nogo receptor 1/p75 neurotrophin receptor, thereby inhibiting axon growth (8).
A search using the National Center for Biotechnology Information database (https://www.ncbi.nlm.nih.gov/) indicated that the AMIGO2 gene is located on the q13.11 antisense chain of chromosome 12 (Fig. 1B). AMIGO2 was initially found to inhibit cell apoptosis and promote survival in cerebellar granule neurons. Furthermore, it mediates cell interactions through homophilic and heterophilic adhesion with AMIGO1 or AMIGO3. In cortical neurons, AMIGO2 mRNA expression depends on its electrical activity. When its electrical activity is suppressed, AMIGO2 mRNA expression decreases (9). Soto et al (10) found that the AMIGO2 protein was expressed in starburst amacrine cells (SACs) and rod bipolar cells (RBCs) of the retina. In vivo and in vitro experiments demonstrated that deletion of AMIGO2 led to dendritic expansion in SACs and RBCs, while dendrites of other neurons remained unaffected, which suggested that AMIGO2 regulated the size and coverage of dendrites in SACs and RBCs (10).
AMIGO2 is also instrumental in the immune system. Previous research has revealed that AMIGO2 mRNA was sharply upregulated in T helper (Th)2 cells but not in Th0 and Th1 cells, where Th2 cells are distinguished from Th0 and Th1 cells (11). This suggests that AMIGO2 may be involved in Th cell differentiation. Th cells with knockdown of AMIGO2 expression exhibit reduced activation and increased proliferation, accompanied by abnormal secretion of pro-inflammatory and anti-inflammatory cytokines (12). A previous study indicated that AMIGO2 expression was increased in rheumatoid arthritis (RA) synoviocytes compared with normal synoviocytes or osteoarthritis synoviocytes after treatment with IL-17A and TNF-α. This suggests that AMIGO2 may be involved in the inflammatory biological activity of RA synoviocytes. Additionally, AMIGO2 expression is regulated by high mobility group box-1 in RA synoviocytes, which synergizes with pro-inflammatory cytokines to upregulate AMIGO2 expression, protecting cells from cadmium-induced apoptosis. These findings indicate the potential involvement of AMIGO2 in the pathogenesis of RA (13).
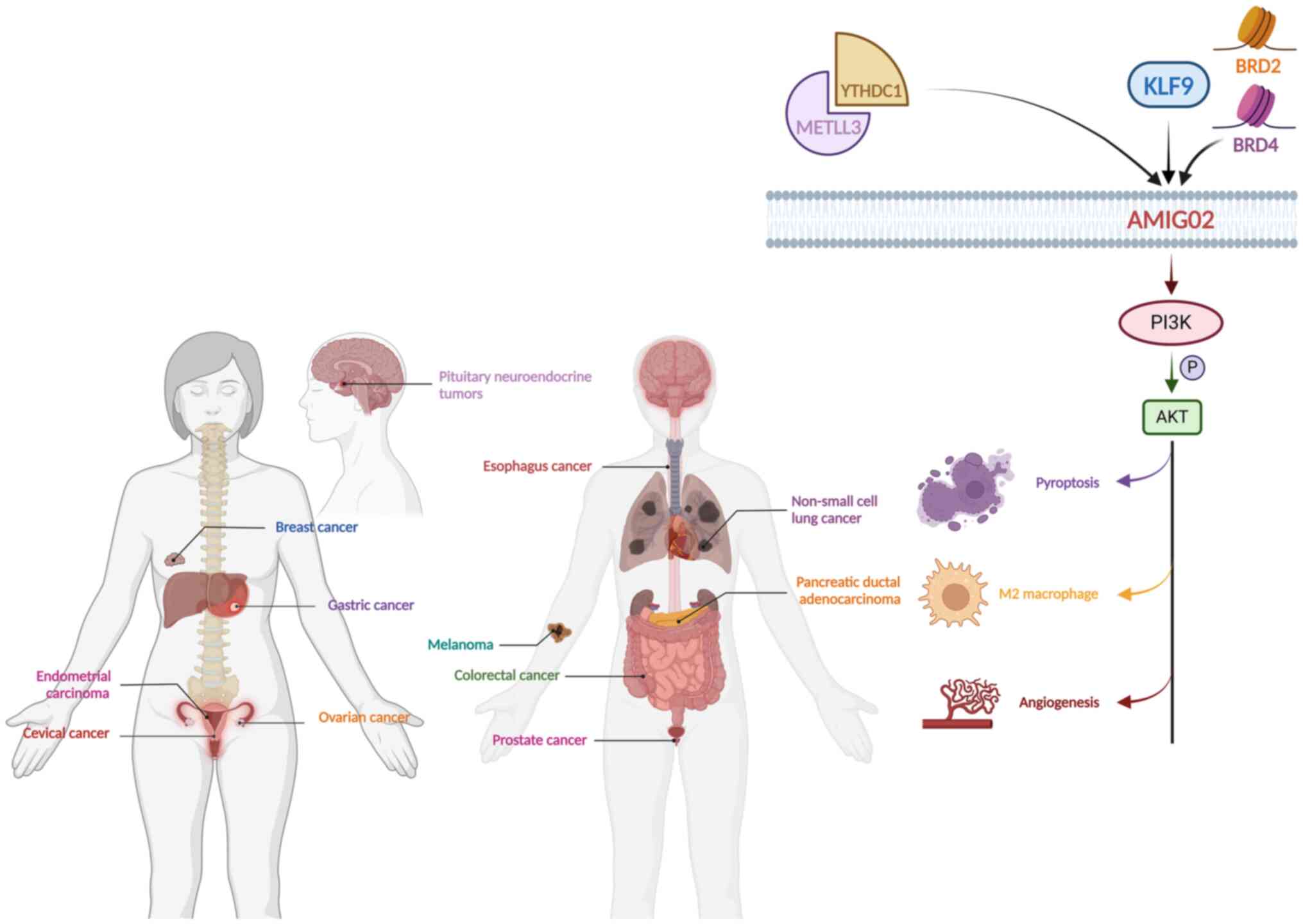
Current research has demonstrated that AMIGO2 can be involved in regulating cancer progression through various pathways and is closely associated with clinical pathological parameters and the prognosis of patients with cancer. Overexpression of AMIGO2 was revealed to promote the proliferation, invasion and adhesion of multiple cancer cells, while downregulation of AMIGO2 was identified to inhibit the tumorigenicity of cancer cells. Furthermore, AMIGO2 has been demonstrated to bind to the pleckstrin homology domain of pyruvate dehydrogenase kinase 1 (PDK1) directly, activating the PDK1-Akt signaling pathway, thereby promoting survival, migration and angiogenesis (14). Additionally, previous studies have demonstrated that the PI3K/PDK1/Akt signaling pathway could promote angiogenesis and accelerate tumor progression mediated by growth factors (15–18). The present review revealed abnormal expression of AMIGO2 in different cancer types and potential molecular mechanisms (Table I; Fig. 2). Therefore, AMIGO2 may be a novel therapeutic target for cancer and angiogenesis-related diseases.
AMIGO2 and cancer
Digestive system tumors
Esophageal cancer, which has an age-standardized incidence of 6.3/100,000 and a mortality rate of 5.6/100,000, is one of the most common digestive system tumors and can be divided into two subtypes: Adenocarcinoma and squamous cell carcinoma (19). Esophageal squamous cell carcinoma (ESCC) is the most common subtype of esophageal cancer. Studies have revealed that dysfunction of RNA N6-methyladenosine (m6A) could lead to the progression of ESCC development. For example, Chen et al (20) found that METTL3 induced ESCC cell metastasis by upregulating glutaminase 2 expression. Li et al (21) confirmed that METTL3 could prompt proliferation and metastasis of ESCC cells by mediating the collagen type XII α1 chain/MAPK signaling pathway. A previous study by the authors explored other underlying mechanisms of METTL3 regulating the biological behaviors of ESCC cells (22). First, the mRNA, protein and mRNA m6A modification levels of AMIGO2 were reduced by METTL3 knockdown. Furthermore, METTL3 knockdown decreased AMIGO2 expression, inhibiting ESCC cell proliferation and migration. Additionally, METTL3 knockdown diminished m6A modification in the 5′-untranslated regions of AMIGO2 precursor mRNA (pre-mRNA). YTH m6A RNA binding protein C1 (YTHDC1) is an m6A reader in the nucleus and can participate in the regulation of gene expression by adjusting alternative splicing of pre-mRNA (23–25). YTHDC1 coordinates with AMIGO2 pre-mRNA to affect the splicing process of AMIGO2 pre-mRNA, which induces downregulation of AMIGO2 expression. However, the downstream target genes of AMIGO2 in ESCC cells remain unclear and require further study.
AMIGO2 has been revealed to serve a positive role in gastric cancer development. Nakamura et al (26) conducted transcriptome analyses, including 14 gastric cancer cell lines, as well as tissues and adjacent tissues of 230 patients, and demonstrated that AMIGO2 expression was positively associated with forkhead box C2, nodal growth differentiation factor and gem nuclear organelle associated protein 2, while it was negatively associated with TFP12. Furthermore, patients with gastric cancer with high AMIGO2 expression tend to have relatively shorter survival. Additionally, CA19-9 expression, tumor location, infiltration and lymph node metastasis are statistically different between patients with high AMIGO2 expression and patients with low AMIGO2 expression. Goto et al (27) revealed that AMIGO2 expression was moderately or vigorously positive in 128 patients with gastric cancer, and predominantly observed in the cytoplasm and nucleus of cancer cells by immunohistochemistry. Clinical pathological features, such as male sex and vascular invasion, were associated with AMIGO2 expression. When compared with patients with gastric cancer with low AMIGO2 expression, patients with high AMIGO2 expression had a higher risk of liver metastasis and peritoneal metastasis, but the difference in AMIGO2 expression did not markedly affect lung and lymph node metastasis. This suggests that upregulation of AMIGO2 may promote liver metastasis and peritoneal metastasis in gastric cancer, while having no impact on lung or lymph node metastasis. Furthermore, AMIGO2 not only influenced gastric cancer cell metastasis and the survival of patients with gastric cancer but also altered the morphology and chromosome number of gastric cancer cells. In a study by Rabenau et al (28), downregulation of AMIGO2 resulted in gastric cancer cell vacuolization, characterized by a five-fold increase in cell volume and an increase in chromosome number. Subsequently, cell adhesion and migration and the ability to form tumors in mice were decreased (28). The aforementioned findings collectively indicate that AMIGO2 serves a regulatory role in gastric cancer and may serve as a prognostic biomarker for gastric cancer.
In another study by Goto et al (29), immunohistochemistry was used to detect the AMIGO2 protein in 173 patients with colorectal carcinoma, revealing that AMIGO2 expression was high in 28.3% of patients with colorectal carcinoma. By contrast, 71.7% of patients exhibited low AMIGO2 expression. Additionally, the AMIGO2 protein was distributed in the nucleus and cytoplasm of colorectal carcinoma cells (29). AMIGO2 is not only upregulated in colorectal carcinoma, but is also associated with colorectal carcinoma cell metastasis. Patients with high AMIGO2 expression are more likely to have liver or lung metastasis, whereas AMIGO2 does not affect peritoneal metastasis. In vitro experiments indicated that AMIGO2 expression was low in Caco-2 cells, whereas it was high in HCT116 cells. After the overexpression of AMIGO2 in Caco-2 cells, proliferation and invasion were enhanced in Caco-2 cells, and adhesion to human hepatic sinusoidal endothelial cells (HHSECs) was also considerably improved. Conversely, AMIGO2 knockdown substantially reduced the proliferation, invasion and adhesion of HCT116 cells. Further investigation revealed that AMIGO2 was more likely to be expressed in differentiated colorectal carcinoma tissues than in undifferentiated colorectal carcinoma tissues, suggesting that AMIGO2 may be associated with the differentiation of tumors (30). These research findings indicate that AMIGO2 is involved in cell proliferation, invasion and adhesion of colorectal carcinoma cells. Goto et al (29) additionally revealed that AMIGO2 may undergo post-translational modification of N-glycosylation, which promoted tumor cell signaling transduction, angiogenesis and metastasis (31). However, it is still uncertain through which molecular mechanism the glycosylation of AMIGO2 affects colorectal carcinoma. Thus, AMIGO2 facilitates the development of colorectal carcinoma and may serve as a promising therapeutic target for colorectal carcinoma.
Research has indicated limited efficacy of immunotherapeutic approaches for patients with pancreatic ductal adenocarcinoma (PDAC) (32,33). Chen et al (34) first observed high AMIGO2 expression in PDAC, and patients with increased AMIGO2 expression exhibited lower overall survival and disease-free survival than patients with low AMIGO2 expression. In addition, the authors found an association between AMIGO2 and M2 macrophage polarization. Both M0 and M2 macrophages were negatively associated with CD8+ T cells in PDAC. It is well known that CD8+ T cells are the main regulators in the antitumor immune response (35,36). Therefore, tumor immunity is impaired when AMIGO2 is highly expressed in patients with PDAC. These observations were further confirmed in in vitro experiments. The proliferation of PDAC cells was inhibited following AMIGO2 knockdown. Given previous research findings suggesting the involvement of AMIGO2 in the Akt pathway (14), Chen et al (34) hypothesized that AMIGO2 may activate M2 macrophage polarization via the Akt pathway, which requires further investigation to confirm.
Liver metastasis is the most common type of metastasis in various digestive system tumors with poor outcomes and prognoses (37). It is essential to identify potential therapeutic targets for liver metastasis. Changes in cell adhesion ability could profoundly affect the tumor metastasis process (38). Izutsu et al (39) observed a positive association between cell adhesion to HHSECs and AMIGO2 expression in gastric and colon cancer cells. Subsequently, the authors revealed that gastric cancer cells with overexpression of AMIGO2 released extracellular vehicles (EVs) containing AMIGO2, and AMIGO2 was transferred to HHSECs via EVs. However, AMIGO2 mRNA expression did not increase in HHSECs, ruling out the possibility of evoking intrinsic AMIGO2 expression. When AMIGO2 protein was delivered into HHSECs, it increased the adhesion to gastric and colon cancer cells, indicating that AMIGO2 could enhance the adhesion of HHSECs to different tumor cells, such as colon and gastric cancer cells. Furthermore, the authors revealed that AMIGO2 did not affect the proliferation and migration of HHSECs (40). Currently, the mechanism by which AMIGO2 strengthens the adhesion of HHSECs to tumor cells requires further investigation, and this could potentially serve as a novel target for liver metastasis.
Kanda et al (41) revealed novel insights regarding the mechanism of liver metastasis in digestive system tumors. The authors screened cells with a high tendency towards liver metastasis from QRSP-11 fibrosarcoma cells, namely LV12 cells, and noticed that the mRNA levels of AMIGO2 were increased in LV12 cells, and AMIGO2 participated in the metastasis process of LV12 cells. When AMIGO2 expression was reduced, the proportion of LV12 cells metastasizing to the liver was decreased. Subsequently, AMIGO2 protein in primary lesions and liver metastases of colon and gastric cancer was detected by immunohistochemistry, and it was revealed that AMIGO2 staining was weakly positive in primary lesions, while it was strongly positive in tumor cells from liver metastases. AMIGO1, AMIGO2 and AMIGO3 expression were also observed in HHSECs but not in pulmonary endothelial cells. Therefore, the authors proposed the mechanism of AMIGO2-induced tumor cell liver metastasis. The cell adhesion of tumor cells to HHSECs may be controlled by homophilic or heterophilic binding of the Amigo family (AMIGO1, AMIGO2 and AMIGO3), which requires further experiments to verify (41). These results demonstrate that AMIGO2 may regulate liver metastasis in digestive system tumors, making it a potential biomarker that is conducive to early detection and treatment.
Urogenital tumors
Han et al (42) reported that AMIGO2 also served an important role in prostate cancer. Downregulation of AMIGO2 could inhibit DU145 and PC3 cell proliferation and colony formation. Survival analysis revealed that patients with high AMIGO2 expression had shorter recurrence-free survival (RFS) times than those with low expression, suggesting that AMIGO2 may be a biomarker for the prediction of RFS in prostate cancer. Further research revealed that differentially expressed genes between groups with high AMIGO2 expression and low AMIGO2 expression were mainly involved in the KRAS and epithelial-mesenchymal transition (EMT) signaling pathways. Knockdown of AMIGO2 expression caused a decrease in Snail, Vimentin and Slug proteins, indicating that AMIGO2 may be involved in the EMT process of prostate cancer cells. Additionally, the upregulation of AMIGO2 expression in prostate cancer cells led to increased resistance to docetaxel (42). In brief, mounting evidence suggests that AMIGO2 may be a potential target for the treatment of prostate cancer. The novel understanding of AMIGO2 could provide a potential theoretical basis and approach for addressing the issue of drug resistance in prostate cancer.
Ovarian cancer frequently presents with peritoneal metastasis (43). To investigate the metastatic dissemination of ovarian cancer, Liu et al (44) cultured an ovarian cancer cell line called IP3, which is characterized by enhanced metastatic ability. In terms of proliferation, there was no discernible difference between IP3 and normal cells. Subsequently, the authors compared the differential gene expression between IP3 and normal cells, identifying that AMIGO2 was the gene with the most marked change in gene expression, and its expression was amplified by 60–65-fold in IP3 cells. To further validate the role of AMIGO2 in IP3 cells, the authors employed CRISPR/CRISPR associated protein 9 technology to knock down AMIGO2 expression. The knockdown resulted in inhibited cell adhesion and migration of IP3 cells, and decreased metastatic ability in vivo. Multi-cellular aggregates (MCAs) were found in the ascites of patients with ovarian cancer, and there was evidence that MCAs were critical to the metastasis of ovarian cancer (45–47). The formation of MCAs involves two steps: First, suspended tumor cells adhere to each other to form loose sheet-like adhesion, and then the sheet-like tumor cells contract into clusters. IP3 cells are compressed into clusters compared with ordinary ovarian cancer cells, while IP3 cells with AMIGO2 knockdown do not exhibit obvious compression into clusters (44), indicating that AMIGO2 regulates the peritoneal metastasis process of ovarian cancer cells and may serve as a novel target for ovarian cancer peritoneal metastasis.
Endometrial cancer is the most common tumor in the female reproductive system (48). Researchers have identified an association between AMIGO2 expression and endometrial cancer development. After upregulation of KLF transcription factor 9 expression in endometrial cancer cells, proteins such as AMIGO2, collagen type IV α1 chain and collagen type IV α2 chain are induced, which subsequently promotes cell adhesion and basement membrane formation (49).
Iida et al (50) revealed that AMIGO2 expression was an independent prognostic factor in cervical cancer recurrence, indicating that AMIGO2 expression was a potential marker for cervical cancer recurrence, which could be used to adjust the treatment strategy.
Respiratory system tumors
Non-small cell lung cancer (NSCLC), which has the highest mortality rate, is the most common type of lung cancer. NSCLC mainly includes subtypes such as squamous cell carcinoma and adenocarcinoma (51). Platinum-based chemotherapy, particularly cisplatin, is commonly used to treat NSCLC (52). However, the sensitivity of tumor cells to cisplatin can influence the potency of chemotherapy. Chen et al (53) revealed that AMIGO2 could reduce the sensitivity of NSCLC cells to cisplatin and modulate the effects of cisplatin on lytic cell death. The authors observed that NSCLC cells treated with cisplatin underwent morphological changes, such as becoming rounded and contracted. Additionally, positive staining for PI revealed the formation of large bubbles originating from the plasma membrane, indicative of pyroptotic morphology. Cisplatin treatment increased the proportion of PI-positive cells exhibiting pyroptotic features. AMIGO2 was found to mitigate the impact of cisplatin on the percentage of PI-positive cells. Specifically, compared with negative control cells, AMIGO2-silenced cells exhibited a more pronounced formation of large bubbles. Furthermore, AMIGO2 likely suppressed cisplatin-induced pyroptosis through modulation of the caspase cascade via activation of the PDK1/Akt (T308) signaling axis. These findings were further validated through in vivo experiments (53).
Other tumors
Breast cancer is one of the most common malignant tumors, with high expression levels of estrogen receptor (ER) and HER2 in breast cancer tissues being well-recognized indicators of patient prognosis (54–56). Shuai et al (57) revealed that AMIGO2 was related to the risk of ER+ and HER2−breast cancer, suggesting that AMIGO2 is a potential prognostic indicator. Nevertheless, Sonzogni et al (58) asserted that increased AMIGO2 expression was unrelated to the prognosis in all types of breast cancer. Additionally, high AMIGO2 expression was associated with low RFS in patients with TP53 wild-type breast cancer, regardless of the state of ER, but exhibited no association with low RFS in patients with TP53 wild-type breast cancer, regardless of the state of HER2 (58). These conclusions from the literature exhibit contradictions, possibly due to the limited number of samples.
AMIGO2 serves a crucial role in regulating melanoma cell functions such as proliferation, clone-forming ability, cell cycle progression and apoptosis. It is upregulated in melanoma cells, including 501MEL and SKme1147 cells. Additionally, AMIGO2 knockdown in melanoma cells reduces proliferation, clone-forming ability and apoptosis with G1/S phase arrest (59). Bromodomain containing (BRD)2 and BRD4, members of the bromodomain and extraterminal domain (BET) family, are highly expressed in melanoma tissues and promote melanoma development. Melanoma progression can be suppressed using BET inhibitors to downregulate BRD2 and BRD4 expression. In previous studies it was revealed that downregulation of BRD2 and BRD4 decreased AMIGO2 expression (60–62). When investigating the interaction between protein tyrosine kinase 7 (PTK7) and AMIGO2, it was found that downregulation of PTK7 hindered proliferation and induced apoptosis in melanoma cells, which could be regulated by protein hydrolysis. MMP1 and a disintegrin and metalloproteinase-17 hydrolyze the PTK7 protein to produce the C-terminal fragment cardiotrophin (CTF)1. γ-Secretase further cleaves CTF1 to generate CTF2, which is transferred to the nucleus (63). Knockdown of AMIGO2 expression leads to the accumulation of CTF1 and CTF2, suggesting that AMIGO2 and PTK7 jointly promote melanoma cell survival, and AMIGO2 can affect PTK7 function by regulating the PTK7 hydrolysis process (59). These studies demonstrate the important role of AMIGO2 in melanoma and its potential clinical implications.
Pituitary neuroendocrine tumors are common intracranial tumors that can secrete harmful hormones, triggering various systemic diseases. Thus, identifying relevant biomarkers for prognosis and treatment analysis is of vital clinical significance. Cui et al (64) observed that AMIGO2 was highly expressed in gonadotroph, somatotroph and lactotroph tumors, implying that AMIGO2 may act as a potential biomarker, which needs further investigation to confirm.
Prospects
Aberrant AMIGO2 expression has been observed to be closely associated with the proliferation, migration, adhesion and apoptosis of tumor cells in numerous tumors. These findings reveal that AMIGO2 is an oncogene influencing tumor initiation and progression. Currently, the understanding of the role of AMIGO2 in tumors is still in its preliminary stage. Although research has been conducted on AMIGO2 expression in tumors and its impact on the biological features of tumor cells, its specific functions in tumor regulation still require further exploration.
EMT is pivotal in embryonic development, tissue remodeling, metastasis and fibrotic diseases. Epithelial-derived malignant tumor cells acquire enhanced migration and invasion abilities during this process (65). Han et al (42) suggested a potential mechanism between AMIGO2 and the regulation of EMT-related proteins, such as Slug and Snail, in prostate cancer. However, further investigation is needed to ascertain whether AMIGO2 can modulate the migration and invasion of other tumor cells via EMT.
PDK1 is a serine/threonine protein kinase belonging to the AGC kinase family and can be activated via signal transduction by extracellular receptors. Upon activation by growth factors binding to receptor tyrosine kinase, PI3K is activated, leading to the conversion of phosphatidylinositol 4,5-bisphosphate to phosphatidylinositol(3,4,5)-trisphosphate (PIP3). Subsequently, PIP3 binds to Akt and PDK1, and PDK1 phosphorylates the Akt protein, activating downstream signaling pathways such as the mTOR and Bax signaling pathways (66,67), consequently influencing various biological behaviors of cells. Studies have revealed that AMIGO2 could regulate M2 macrophage polarization, angiogenesis and chemotherapy resistance by affecting the PI3K/AKT signaling pathway (14,36,53). Nevertheless, the specific molecular mechanisms underlying the interaction between AMIGO2 and PI3K/AKT signaling remain unclear and warrant further exploration.
Some researchers have proposed that AMIGO2 may serve as a potential biomarker for cancer diagnosis and prognosis. However, the evidence supporting this claim is currently insufficient and requires substantial clinical and experimental data for validation. In summary, the present review delves into the research on AMIGO2 in various cancer types, enhancing the comprehension of cancer pathogenesis and offering novel insights for the development of novel biomarkers and personalized cancer therapies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
ZT performed the literature review and wrote the paper. DZ was responsible for reviewing the literature and revising the final paper. RJ and BZ were involved in drafting the manuscript and creating the table and figures. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Ruan Y, Chen L, Xie D, Luo T, Xu Y, Ye T, Chen X, Feng X and Wu X: Mechanisms of cell adhesion molecules in endocrine-related cancers: A concise outlook. Front Endocrinol (Lausanne). 13:8654362022. View Article : Google Scholar : PubMed/NCBI | |
|
Makrilia N, Kollias A, Manolopoulos L and Syrigos K: Cell adhesion molecules: Role and clinical significance in cancer. Cancer Invest. 27:1023–1037. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Kim HN, Ruan Y, Ogana H and Kim YM: Cadherins, selectins, and integrins in CAM-DR in leukemia. Front Oncol. 10:5927332020. View Article : Google Scholar : PubMed/NCBI | |
|
Windisch R, Pirschtat N, Kellner C, Chen-Wichmann L, Lausen J, Humpe A, Krause DS and Wichmann C: Oncogenic deregulation of cell adhesion molecules in leukemia. Cancers (Basel). 11:3112019. View Article : Google Scholar : PubMed/NCBI | |
|
Hassn Mesrati M, Syafruddin SE, Mohtar MA and Syahir A: CD44: A multifunctional mediator of cancer progression. Biomolecules. 11:18502021. View Article : Google Scholar : PubMed/NCBI | |
|
Kuja-Panula J, Kiiltomäki M, Yamashiro T, Rouhiainen A and Rauvala H: AMIGO, a transmembrane protein implicated in axon tract development, defines a novel protein family with leucine-rich repeats. J Cell Biol. 160:963–973. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Soto F, Shen N and Kerschensteiner D: AMIGO1 promotes axon growth and territory matching in the retina. J Neurosci. 42:2678–2689. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Ahmed Z, Douglas MR, John G, Berry M and Logan A: AMIGO3 is an NgR1/p75 co-receptor signalling axon growth inhibition in the acute phase of adult central nervous system injury. PLoS One. 8:e618782013. View Article : Google Scholar : PubMed/NCBI | |
|
Ono T, Sekino-Suzuki N, Kikkawa Y, Yonekawa H and Kawashima S: Alivin 1, a novel neuronal activity-dependent gene, inhibits apoptosis and promotes survival of cerebellar granule neurons. J Neurosci. 23:5887–5896. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Soto F, Tien NW, Goel A, Zhao L, Ruzycki PA and Kerschensteiner D: AMIGO2 scales dendrite arbors in the retina. Cell Rep. 29:1568–1578.e4. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Lund RJ, Löytömäki M, Naumanen T, Dixon C, Chen Z, Ahlfors H, Tuomela S, Tahvanainen J, Scheinin J, Henttinen T, et al: Genome-wide identification of novel genes involved in early Th1 and Th2 cell differentiation. J Immunol. 178:3648–3660. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Li Z, Khan MM, Kuja-Panula J, Wang H, Chen Y, Guo D, Chen ZJ, Lahesmaa R, Rauvala H and Tian L: AMIGO2 modulates T cell functions and its deficiency in mice ameliorates experimental autoimmune encephalomyelitis. Brain Behav Immun. 62:110–123. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Benedetti G, Bonaventura P, Lavocat F and Miossec P: IL-17A and TNF-α increase the expression of the antiapoptotic adhesion molecule Amigo-2 in arthritis synoviocytes. Front Immunol. 7:2542016. View Article : Google Scholar : PubMed/NCBI | |
|
Park H, Lee S, Shrestha P, Kim J, Park JA, Ko Y, Ban YH, Park DY, Ha SJ, Koh GY, et al: AMIGO2, a novel membrane anchor of PDK1, controls cell survival and angiogenesis via Akt activation. J Cell Biol. 211:619–637. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Yu X, Wu T, Liao B, Du Z and Zhu P: Anticancer potential of corilagin on T24 and TSGH 8301 bladder cancer cells via the activation of apoptosis by the suppression of NF-κB-induced P13K/Akt signaling pathway. Environ Toxicol. 37:1152–1159. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Saahene RO, Agbo E, Barnes P, Yahaya ES, Amoani B, Nuvor SV and Okyere P: A review: Mechanism of phyllanthus urinaria in Cancers-NF-κB, P13K/AKT, and MAPKs signaling activation. Evid Based Complement Alternat Med. 2021:45143422021. View Article : Google Scholar : PubMed/NCBI | |
|
Zheng H and Liu JF: Studies on the relationship between P13K/AKT signal pathway-mediated MMP-9 gene and lung cancer. Eur Rev Med Pharmacol Sci. 21:753–759. 2017.PubMed/NCBI | |
|
Chen ZF, Wang J, Yu Y and Wei W: MicroRNA-936 promotes proliferation and invasion of gastric cancer cells by down-regulating FGF2 expression and activating P13K/Akt signaling pathway. Eur Rev Med Pharmacol Sci. 24:6707–6715. 2020.PubMed/NCBI | |
|
Zhao YX, Zhao HP, Zhao MY, Yu Y, Qi X, Wang JH and Lv J: Latest insights into the global epidemiological features, screening, early diagnosis and prognosis prediction of esophageal squamous cell carcinoma. World J Gastroenterol. 30:2638–2656. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Chen X, Huang L, Yang T, Xu J, Zhang C, Deng Z, Yang X, Liu N, Chen S, Lin S, et al: METTL3 Promotes esophageal squamous cell carcinoma metastasis through enhancing GLS2 expression. Front Oncol. 11:6674512021. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, Li Z, Xu Y, Huang C and Shan B: METTL3 facilitates tumor progression by COL12A1/MAPK signaling pathway in esophageal squamous cell carcinoma. J Cancer. 13:1972–1984. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Qiu Y, Tian Z, Miao TY, Shen L, Chen J, Li PF, Zhu ZX, Zhu ZF, Wu WJ, Xu X and Shen WG: The METTL3-m6A-YTHDC1-AMIGO2 axis contributes to cell proliferation and migration in esophageal squamous cell carcinoma. Gene. 908:1482812024. View Article : Google Scholar : PubMed/NCBI | |
|
Jiang X, Liu B, Nie Z, Duan L, Xiong Q, Jin Z, Yang C and Chen Y: The role of m6A modification in the biological functions and diseases. Signal Transduct Target Ther. 6:742021. View Article : Google Scholar : PubMed/NCBI | |
|
Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, Sun HY, Li A, Ping XL, Lai WY, et al: Nuclear m(6)A Reader YTHDC1 Regulates mRNA Splicing. Mol Cell. 61:507–519. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Roundtree IA, Luo GZ, Zhang Z, Wang X, Zhou T, Cui Y, Sha J, Huang X, Guerrero L, Xie P, et al: YTHDC1 mediates nuclear export of N6-methyladenosine methylated mRNAs. Elife. 6:e313112017. View Article : Google Scholar : PubMed/NCBI | |
|
Nakamura S, Kanda M, Shimizu D, Tanaka C, Inokawa Y, Hattori N, Hayashi M, Yamada S, Nakayama G, Omae K, et al: AMIGO2 Expression as a potential prognostic biomarker for gastric cancer. Anticancer Res. 40:6713–6721. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Goto K, Morimoto M, Osaki M, Tanio A, Izutsu R, Fujiwara Y and Okada F: The impact of AMIGO2 on prognosis and hepatic metastasis in gastric cancer patients. BMC Cancer. 22:2802022. View Article : Google Scholar : PubMed/NCBI | |
|
Rabenau KE, O'Toole JM, Bassi R, Kotanides H, Witte L, Ludwig DL and Pereira DS: DEGA/AMIGO-2, a leucine-rich repeat family member, differentially expressed in human gastric adenocarcinoma: Effects on ploidy, chromosomal stability, cell adhesion/migration and tumorigenicity. Oncogene. 23:5056–5067. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Goto K, Osaki M, Izutsu R, Tanaka H, Sasaki R, Tanio A, Satofuka H, Kazuki Y, Yamamoto M, Kugoh H, et al: Establishment of an antibody specific for AMIGO2 improves immunohistochemical evaluation of liver metastases and clinical outcomes in patients with colorectal cancer. Diagn Pathol. 17:162022. View Article : Google Scholar : PubMed/NCBI | |
|
Tanio A, Saito H, Amisaki M, Hara K, Sugezawa K, Uejima C, Tada Y, Kihara K, Yamamoto M, Nosaka K, et al: AMIGO2 as a novel indicator of liver metastasis in patients with colorectal cancer. Oncol Lett. 21:2782021. View Article : Google Scholar : PubMed/NCBI | |
|
Thomas D, Rathinavel AK and Radhakrishnan P: Altered glycosylation in cancer: A promising target for biomarkers and therapeutics. Biochim Biophys Acta Rev Cancer. 1875:1884642021. View Article : Google Scholar : PubMed/NCBI | |
|
Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, Sherry RM, Topalian SL, Yang JC, Lowy I and Rosenberg SA: Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 33:828–833. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al: Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Chen S, Du W, Feng K, Liu K, Li C, Li S and Yin H: AMIGO2 is a pivotal therapeutic target related to M2 polarization of macrophages in pancreatic ductal adenocarcinoma. Aging (Albany NY). 16:1111–1127. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Kishton RJ, Sukumar M and Restifo NP: Metabolic regulation of T cell longevity and function in tumor immunotherapy. Cell Metab. 26:94–109. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
MacNabb BW, Tumuluru S, Chen X, Godfrey J, Kasal DN, Yu J, Jongsma MLM, Spaapen RM, Kline DE and Kline J: Dendritic cells can prime anti-tumor CD8(+) T cell responses through major histocompatibility complex cross-dressing. Immunity. 55:982–997.e8. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R and Abbruzzese JL: Metastatic patterns in adenocarcinoma. Cancer. 106:1624–1633. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Hanley WD, Burdick MM, Konstantopoulos K and Sackstein R: CD44 on LS174T colon carcinoma cells possesses E-selectin ligand activity. Cancer Res. 65:5812–5817. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Izutsu R, Osaki M, Jehung JP, Seong HK and Okada F: Liver metastasis formation is defined by AMIGO2 expression via adhesion to hepatic endothelial cells in human gastric and colorectal cancer cells. Pathol Res Pract. 237:1540152022. View Article : Google Scholar : PubMed/NCBI | |
|
Izutsu R, Osaki M, Nemoto H, Jingu M, Sasaki R, Yoshioka Y, Ochiya T and Okada F: AMIGO2 contained in cancer cell-derived extracellular vesicles enhances the adhesion of liver endothelial cells to cancer cells. Sci Rep. 12:7922022. View Article : Google Scholar : PubMed/NCBI | |
|
Kanda Y, Osaki M, Onuma K, Sonoda A, Kobayashi M, Hamada J, Nicolson GL, Ochiya T and Okada F: Amigo2-upregulation in tumour cells facilitates their attachment to liver endothelial cells resulting in liver metastases. Sci Rep. 7:435672017. View Article : Google Scholar : PubMed/NCBI | |
|
Han Z, Feng Y, Deng Y, Tang Z, Cai S, Zhuo Y, Liang Y, Ye J, Cai Z, Yang S, et al: Integrated analysis reveals prognostic value and progression-related role of AMIGO2 in prostate cancer. Transl Androl Urol. 11:914–928. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Lengyel E: Ovarian cancer development and metastasis. Am J Pathol. 177:1053–1064. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Y, Yang J, Shi Z, Tan X, Jin N, O'Brien C, Ott C, Grisoli A, Lee E, Volk K, et al: In vivo selection of highly metastatic human ovarian cancer sublines reveals role for AMIGO2 in intra-peritoneal metastatic regulation. Cancer Lett. 503:163–173. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Burleson KM, Casey RC, Skubitz KM, Pambuccian SE, Oegema TR Jr and Skubitz AP: Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecol Oncol. 93:170–181. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
L'Espérance S, Bachvarova M, Tetu B, Mes-Masson AM and Bachvarov D: Global gene expression analysis of early response to chemotherapy treatment in ovarian cancer spheroids. BMC Genomics. 9:992008. View Article : Google Scholar : PubMed/NCBI | |
|
Shield K, Ackland ML, Ahmed N and Rice GE: Multicellular spheroids in ovarian cancer metastases: Biology and pathology. Gynecol Oncol. 113:143–148. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Koskas M, Amant F, Mirza MR and Creutzberg CL: Cancer of the corpus uteri: 2021 update. Int J Gynaecol Obstet. 155 (Suppl 1):S45–S60. 2021. View Article : Google Scholar | |
|
Simmen FA, Su Y, Xiao R, Zeng Z and Simmen RC: The Krüppel-like factor 9 (KLF9) network in HEC-1-A endometrial carcinoma cells suggests the carcinogenic potential of dys-regulated KLF9 expression. Reprod Biol Endocrinol. 6:412008. View Article : Google Scholar : PubMed/NCBI | |
|
Iida Y, Osaki M, Sato S, Izutsu R, Seong H, Okawa M, Osaku D, Komatsu H, Taniguchi F, Okada F, et al: AMIGO2 expression as a predictor of recurrence in cervical cancer with intermediate risk. Mol Clin Oncol. 19:562023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Y, Chen Y, Lu LL, Xie XL, Huan R, Wu LF, Tan LN, Xu T and Jin Y: The role and therapeutic potential of Non-coding RNAs in resistance to EGFR-TKIs targeted therapy for Non-small cell lung cancer. Curr Med Chem. Feb 16–2024.doi: 10.2174/0109298673275752231219080500 (Epub ahead of print). | |
|
Duma N, Santana-Davila R and Molina JR: Non-Small cell lung cancer: Epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 94:1623–1640. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Chen LK, Lin SP, Xie YH, Tan XP, Xiong BH, Zeng XF, Zhu CR, Cao SY, Ye XY, Liu HJ and Wu XP: AMIGO2 attenuates innate cisplatin sensitivity by suppression of GSDME-conferred pyroptosis in non-small cell lung cancer. J Cell Mol Med. 27:2412–2423. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Arciero CA, Guo Y, Jiang R, Behera M, O'Regan R, Peng L and Li X: ER+/HER2+ breast cancer has different metastatic patterns and better survival than ER-/HER2+ breast cancer. Clin Breast Cancer. 19:236–245. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
van de Ven S, Smit VT, Dekker TJ, Nortier JW and Kroep JR: Discordances in ER, PR and HER2 receptors after neoadjuvant chemotherapy in breast cancer. Cancer Treat Rev. 37:422–430. 2011.PubMed/NCBI | |
|
Najjar S and Allison KH: Updates on breast biomarkers. Virchows Arch. 480:163–176. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Shuai C, Yuan F, Liu Y, Wang C, Wang J and He H: Estrogen receptor-positive breast cancer survival prediction and analysis of resistance-related genes introduction. PeerJ. 9:e122022021. View Article : Google Scholar : PubMed/NCBI | |
|
Sonzogni O, Haynes J, Seifried LA, Kamel YM, Huang K, BeGora MD, Yeung FA, Robert-Tissot C, Heng YJ, Yuan X, et al: Reporters to mark and eliminate basal or luminal epithelial cells in culture and in vivo. PLoS Biol. 16:e20040492018. View Article : Google Scholar : PubMed/NCBI | |
|
Fontanals-Cirera B, Hasson D, Vardabasso C, Di Micco R, Agrawal P, Chowdhury A, Gantz M, de Pablos-Aragoneses A, Morgenstern A, Wu P, et al: Harnessing BET inhibitor sensitivity reveals AMIGO2 as a melanoma survival gene. Mol Cell. 68:731–744.e9. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Gallagher SJ, Mijatov B, Gunatilake D, Tiffen JC, Gowrishankar K, Jin L, Pupo GM, Cullinane C, Prinjha RK, Smithers N, et al: The epigenetic regulator I-BET151 induces BIM-dependent apoptosis and cell cycle arrest of human melanoma cells. J Invest Dermatol. 134:2795–2805. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Vardabasso C, Gaspar-Maia A, Hasson D, Pünzeler S, Valle-Garcia D, Straub T, Keilhauer EC, Strub T, Dong J, Panda T, et al: Histone variant H2A.Z.2 mediates proliferation and drug sensitivity of malignant melanoma. Mol Cell. 59:75–88. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Segura MF, Fontanals-Cirera B, Gaziel-Sovran A, Guijarro MV, Hanniford D, Zhang G, González-Gomez P, Morante M, Jubierre L, Zhang W, et al: BRD4 sustains melanoma proliferation and represents a new target for epigenetic therapy. Cancer Res. 73:6264–6276. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Na HW, Shin WS, Ludwig A and Lee ST: The cytosolic domain of protein-tyrosine kinase 7 (PTK7), generated from sequential cleavage by a disintegrin and metalloprotease 17 (ADAM17) and γ-secretase, enhances cell proliferation and migration in colon cancer cells. J Biol Chem. 287:25001–25009. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Cui Y, Li C, Jiang Z, Zhang S, Li Q, Liu X, Zhou Y, Li R, Wei L, Li L, et al: Single-cell transcriptome and genome analyses of pituitary neuroendocrine tumors. Neuro Oncol. 23:1859–1871. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Dongre A and Weinberg RA: New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 20:69–84. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Porter AC and Vaillancourt RR: Tyrosine kinase receptor-activated signal transduction pathways which lead to oncogenesis. Oncogene. 17:1343–1352. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Tamgüney T, Zhang C, Fiedler D, Shokat K and Stokoe D: Analysis of 3-phosphoinositide-dependent kinase-1 signaling and function in ES cells. Exp Cell Res. 314:2299–2312. 2008. View Article : Google Scholar : PubMed/NCBI |